Biomineralization on single crystalline rutile: the modulated growth of hydroxyapatite by fibronectin in a simulated body fluid
Abstract
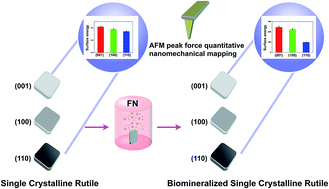
The aim of this study is to probe the complex interaction between surface bioactivity and protein adsorption on single crystalline rutile. Our previous studies have shown that single crystalline rutile possessed in vitro bioactivity and the crystalline faces affected the hydroxyapatite (HA) formation. However, upon implantation, a fast adsorption of proteins, from the biological fluids, is intermediated by a water layer towards the biomaterial interface. Thus the effect of protein on the bioactivity must be addressed. In this study, the HA growth dynamics on (001), (100) and (110) faces was investigated in a simulated body fluid with the presence of fibronectin (FN) by two different processes. The surface adhesion of each face before and after FN adsorption, as revealed by direct numerical values, was determined by atomic force microscopy (AFM) based peak force quantitative nanomechanical mapping (PF-QNM) for the first time. The findings suggest the surface energies of FN pre-adsorbed (001), (100) and (110) faces have been enhanced, leading to the subsequent accelerated HA formation. Furthermore, (001) and (100) faces were found to have larger coverage of HA crystals than (110) face at an early stage. In addition, various characterizations were performed to probe the chemical and crystal structures of as-grown biomimetic HA crystals, and in particular, the Ca/P ratio variations at different soaking time points.


 Please wait while we load your content...
Please wait while we load your content...