Constrained azeotropic optimization of extraction system components for the safe and efficient recovery of a desired metabolite (e.g., 3-demethylated colchicine)†
Abstract
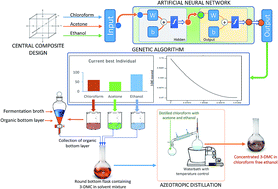
This study suggests an efficient strategy for the maximal extraction of human consumable metabolites in a safe and non-toxic solvent system for the cases where the best extraction solvent belongs to the restricted class (ICH, Quality Guidelines). An optimum composition of extraction system components (chloroform : acetone : ethyl alcohol) was determined for the maximum and chloroform free recovery of 3-demethylated colchicine (3-DMC) from a fermentation broth. A feed forward backprop artificial neural network (ANN) was trained and validated to establish a mathematical relationship between the inputs (volumes of extraction system components) and outputs (concentration of 3-DMC). Inputs to the ANN were kept under strict constraints, always satisfying the azeotropic ratio for chloroform : acetone with an excess of ethyl alcohol forming a complex ternary azeotrope. A genetic algorithm (GA) with linear equality matrices defining ternary azeotropic constraints was implemented to perform an exhaustive search for the global optimum composition of the extraction system components with the validated ANN as the selection function. Since, this strategy only allows for the azeotropic ratio, the global optimum is a typical azeotrope with maximum 3-DMC recovery. The purpose of the azeotrope is to safely distil out all the chloroform after extraction, by performing an azeotropic distillation at a lower temperature (63.2 °C) leaving only an excess of ethyl alcohol, which boils at a higher temperature (78 °C). The final optimized volumes of chloroform: 63.055 mL, acetone: 44.138 and ethanol: 92.807 mL resulted in a maximum recovery of 6.5 g L−1 of 3-DMC. This approach was also tested successfully for the energy efficient recovery of betulinic acid in a Class 3 solvent.

 Please wait while we load your content...
Please wait while we load your content...