DOI:
10.1039/C6RA04271F
(Paper)
RSC Adv., 2016,
6, 43463-43469
Synthesis and characterization of hydrophobic association hydrogels with tunable mechanical strength
Received
17th February 2016
, Accepted 26th April 2016
First published on 28th April 2016
Abstract
Designing hydrogels with tunable mechanical properties and self-healing effects is crucial to a variety of applications, such as bioremediation carriers. Here, we synthesized a series of hydrophobic associated hydrogels (HA-gels) through micellar copolymerization in sodium dodecyl sulfate (SDS) aqueous solution. The hydrophobic monomer used is fatty alcohol polyoxyethylene acrylate (AEO-AC), which is significantly more eco-friendly than the traditional octylphenol polyoxyethylene acrylate (OP-AC) hydrophobic monomer. Interestingly, the mechanical properties of HA-gels can be tuned controllably by varying the ratio of AEO-AC-10-5 to AEO-AC-13-5 (AEO-AC-n-5: CnH2n+1(OCH2CH2)5O–C(O)CHCH2; n = 10, 13). The longer and sheer straight carbon chain of AEO-AC-13-5 leads to stronger hydrophobic association point, while the shorter and branched carbon chain of AEO-AC-10-5 results in weaker hydrophobic association point. The resulting AEO-AC-13-5-AM gels possess tough mechanical strength (maximum broken stress is 318 kPa) and high elongation (1000–3000%). Then, we could tune the swelling and stress relaxation behaviors by varying the ratio of SDS to AEO-AC and obtain HA-gels that maintain their shapes in water nearly half a year, with low content of SDS. Lastly, our HA-gels also exhibit good self-healing capability, and offer great opportunities for a lot of prospective biomaterials.
Introduction
Hydrogels are polymeric networks that absorb and retain large amounts of water while maintaining their morphology. Hydrogels have been considered as major candidates for bioremediation carrier materials.1–3 Due do their good biocompatibility and multi-functional practicability. Since Miguel F. Refojo found the secondary hydrophobic structure of poly(2-hydroxyehtyl methacrylate) homogeneous hydrogel in 1967,4 researchers have taken tremendous efforts on fabricating variety of hydrogel structures using different chemical or physical interaction, such as covalently-crosslinked,5 electrostatic effect,6,7 hydrogen bond,8,9 host–guest molecules,10,11 biorecognition,12 hydrophobic effect,13,14 metal-coordination interaction,15,16 constructing double-network,17,18 multicomponent crosslinking etc.19,20 The various structures endow hydrogels with particular performances, reversible thermo-response,21,22 pH response,23,24 ions response.25,26 However, it is difficult to obtain hydrogels with tunable property and self-healing capability resulting from their permanent crosslinking structures. Moreover, the complicated synthesis processes hinder their industrial and biomedical applications.
Hydrophobic association hydrogels (HA-gels) have unusual tough mechanical properties and high elongation. The mechanical properties of HA-gels can be tuned easily by adjusting the ratio of surfactant to hydrophobic monomers.27 The ability of HA-gels to self-heal depends on the equilibrium of “disassociation and re-association” in hydrophobic associated points.28 In previous studies, a variety of hydrophobic monomers were researched: N-isopropyl acrylamide,29 methacrylicacid,30 octadecyl acrylate,31 butylacrylate,32 ε-caprolactone.33 According to these studies, the stronger hydrophobic effect the hydrophobic monomers have, the more difficult they are homogeneously dispersed in water. It is well known that an ideal kind of hydrophobic monomers should have strong hydrophobic effect and could disperse uniformly in the prepared solution. In our previous works octylphenol polyoxyethylene acrylate (OP-AC) was synthesized as hydrophobic monomer, which has both hydrophilic group and hydrophobic group.27,28,34–38 In OP-AC, octylphenol offers strong hydrophobic effect, while polyoxyethylene provides water solubility. Nonetheless, hydrolysed OP-AC is hard-biodegraded and toxicant. Therefore, we attempt to replace OP-AC with fatty alcohol polyoxyethylene acrylate (AEO-AC) to prepare eco-friendly HA-gels.
In present paper, hydrophobic monomer AEO-AC-10-5 and AEO-AC-13-5 were synthesized for the first time through acyl chlorination. AEO-AC-10-5 monomers have various branched alkyl chains, while AEO-AC-13-5 monomers possess sheer straight alkyl chains. There are huge differences between ‘branched gel’ (AEO-AC-10-5-AM gels) and ‘straight gel’ (AEO-AC-13-5-AM gels) in mechanical properties, swelling behaviours and stress relaxation behaviours. As a result, we get a strategy to prepare tunable hydrogels through changing the ratio of two hydrophobic monomers: ‘straight AEO-AC’ and ‘branched AEO-AC’.
Results and discussion
IR analysis of AEO & AEO-AC
FT-IR spectral analysis was employed to confirm the introduction of hydrophobic monomer AEO-AC-m-5 and the results are shown in Fig. 1. From the spectrum of AEO, distinctive adsorption broad peak appears at 3473 cm−1 (axial stretching vibration of O–H). In contrast, the above-mentioned peak disappears in the IR spectrum of AEO-AC. Moreover, the following peaks are observed for AEO-AC: the peak at 1731 cm−1 corresponding to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O; the peak at 1640 cm−1 corresponding to stretching vibration of C
O; the peak at 1640 cm−1 corresponding to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; the peak at 1410 cm−1 corresponding to the shear-vibration of
C; the peak at 1410 cm−1 corresponding to the shear-vibration of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and the peak at 1195 cm−1 corresponding to the asymmetric stretching vibration of C–O–C. All these evidences demonstrate that AEO-AC was successfully synthesized.
CH2 and the peak at 1195 cm−1 corresponding to the asymmetric stretching vibration of C–O–C. All these evidences demonstrate that AEO-AC was successfully synthesized.
 |
| | Fig. 1 FT-IR spectra of AEO and AEO-AC. | |
Effect of type and content of AEO-AC in HA-gels on mechanical properties
As shown in Fig. 2, the elastic modulus increases with the increase of AEO-AC content, but the elongation at break decreases monotonically. The maximum broken stress of AEO-AC-13-5-AM-(AEO 3%) gels is nearly four times greater than that of AEO-AC-10-5-AM-(AEO 3%). It is supposed that all of the hydrophobic monomers are solubilized equally in sufficient SDS micelles. Therefore, as the amount of SDS micelles is constant, the association point's strength becomes stronger with the increase in hydrophobic monomers. AEO-AC-13-5 possesses n-dodecyl or n-tetradecyl straight hydrophobic carbon chains which is similar to sodium dodecyl sulphate. AEO-AC-10-5 possesses short branched carbon chains with larger space steric hindrance. Consequently, the association points in AEO-AC-13-5-AM-(AEO 3%) gels are more compact and stronger than those in AEO-AC-10-5-AM-(AEO 3%) gels. In the uniaxial tensile test, segments of AEO-AC-10-5-AM-(AEO 3%) gels are more easily extracted from the association points, and therefore the maximum stress of the gels is lower. Segments of AEO-AC-13-5-AM-(AEO 3%) gels are difficultly extracted from the association points, so the maximum stress of the gels is higher.
 |
| | Fig. 2 Stress–strain curves of HA-gels with different content and type of AEO-AC in AEO-AC-n-5-AM. | |
Effect of content of SDS in HA-gels on mechanical properties
Fig. 3a illustrates the trend of the maximum broken stress of AEO-AC-10-5-AM-(SDS-R) gels with firstly increasing and then decreasing while increasing R values. According to the previous results from our group,27 HA-gels have a most evenly network structure which shows a maximum mechanical strength at an optimal R value. The two synthesized hydrophobic monomers are not soluble in water without SDS. When the hydrophobic monomer content is constant, the increasing SDS will bring two aspects of effects. Firstly, it decreases the content of hydrophobic monomers in each SDS micelle that weaken the strength of hydrophobic association points; secondly, it increases the number of hydrophobic association domains (Scheme 1). Meanwhile abundant SDS will bring free micelles which are like lubricant resulting in molecular chains moving easily. Therefore, the results in Fig. 3 describe these two synergistic effects by increasing SDS amount. The best R value of AEO-AC-10-5-AM-(SDS-R) gels appears in the vicinity of 2.0, while it is around 3.0 in the case of AEO-AC-13-5-AM-(SDS-R) gels, reflecting the stronger solubilization capacity of AEO-AC-13-5 in SDS micelles. After the optimal R value, the former effect is dominant, and before that the latter effect plays a major role.
 |
| | Fig. 3 Stress–strain curves of AEO-AC-n-m-AM-(SDS-R). (a) AEO-AC-10-5-AM-(SDS-R), (b) AEO-AC-13-5-AM-(SDS-R). | |
 |
| | Scheme 1 When the content of SDS is double, the amount of hydrophobic domain increases and the number of hydrophobic monomers in each SDS micelle decreases. | |
In Fig. 3a, the elongation at break increases steadily with increasing SDS. Whereas the elongation in Fig. 3b goes “long–short–long” tendency with the increase in R value. Short branched AEO-AC-10-5-AM gels processes weaker association points, then segment is easy to be extracted and comes into new association points easily. The elongation becomes longer when increasing SDS because the association points are weaker and the amount of hydrophobic association domains is less. However, in AEO-AC-13-5-AM gels the combination of hydrophobic association point is stronger and the hydrophobic segment is difficult to be extracted from the associated point. When R = 0.5, the amount of SDS is the least and the network is unevenly; R = 1.0, the molecular weight between effective association points is larger because the number of association domains is less, and more long chains appear in HA-gels; R = 2.0, short chains in HA-gels is increasing so there is a sudden decrease in elongation; R = 3.0, 4.0, abundant SDS bring free micelles which lead to longer elongations.
Stress relaxation behaviours
Fig. 4 is stress relaxation curves of two kinds of the HA-gels. The content of AEO-AC monomer is constant (1% mol to AM). The measurement is performed within 2 hours, because the HA-gels will lose water over 2 hours. The results show that the stress curves undergo a quick dropping, then tend to a constant value. For the HA-gels containing AEO-AC-10-5 (Fig. 4), gel relaxation degree increases with the increase of SDS concentration. The maximum relaxation degree of AEO-AC-13-5 (R = 4.0) is 48% in two hours. Under the continuing external force, the hydrophobic fragment in AEO-AC-10-5-AM gels is more easily extracted from the hydrophobic association domain, but the hydrophobic fragment in AEO-AC-13-5-AM gels is difficultly extracted from the hydrophobic association domain due to the much stronger hydrophobic association. Therefore, the AEO-AC-13-5-AM gels are difficult to relax to zero. This also reflects that association capability of AEO-AC-13-5-AM is stronger than that of AEO-AC-10-5-AM.
 |
| | Fig. 4 The stress relaxation curves of HA-gels (the content of AEO-AC is 1 mol% to AM). | |
Swelling behaviours of HA-gels
Fig. 5 shows the swelling behaviour of HA-gels containing the hydrophobic monomers of AEO-AC-10-5 or AEO-AC-13-5. In Fig. 5a, with the increase of SDS content in AEO-AC-10-5-AM (SDS-R), the time lives of HA-gels in water decreases rapidly, and HA-gels ultimately become sol. At final moment, the HA-gels are totally dissolved in water with swelling ratio and remaining gel fraction are equal to zero. In particular, when SDS is equal to 4, the HA-gels can only exist in water for four days. The maximum swelling ratio of AEO-AC-10-5-AM (SDS-R) gels could reach 100 times of the initial HA-gels. Being obvious different, in Fig. 5b, AEO-AC-13-5-AM HA-gels can survive in water for more than 120 days, and the swelling ratio is only about 50 times higher than the initial HA-gels, while the remaining gel fractions show little decrease. As R is equal to 0.5 and 1, the prepared HA-gels could keep their shape and dissolved in water after 160 days, known as ‘permanent hydrogel’. When the HA-gels were placed in distilled water, SDS would come out from the HA-gels due to the presence of the osmotic pressure difference, and water would enter into the HA-gels and make them swelling. Therefore, when the content of SDS increases, the osmotic pressure difference goes up, so the exchange between SDS and water becomes faster and accelerates the swelling process. As previously discussed, the association in AEO-AC-10-5-AM is weak, so the molecular chain is easily extracted from the associated point in the swelling process, lowering the strength of associated points, and causing inability to maintain the HA-gel network. As the state of hydrophobic association domain in water is dynamic balanced, the hydrophobic end of the molecular chain extracted could also interact with the hydrophobic end of other molecular chain and reform new association points. However, if the swelling ratio is too high, the collision probability of molecular chain drawn out will be very low, so new hydrophobic domains will not be formed. Thus the AEO-AC-10-5-AM gels can be quickly dissolved in water and its swelling ratio is large. The molecule chain of AEO-AC-13-5 is difficult to be extracted and can keep their gels forms for a long time due to having stronger hydrophobic association.
 |
| | Fig. 5 The relationship of Sr and GF with swelling time (AEO-AC is 1 mol% to AM and R value varies). | |
Adjustment of mechanical strength of HA-gels
During experiments, two kinds of AEO-AC monomers (AEO-AC-10-5, AEO-AC-13-5) were used to construct HA-gels with a broad range of mechanical strength. Fig. 6 depicts the mechanical properties of HA-gels. Generally, as the content of AEO-AC-10-5 monomers increases the elastic modulus decreases linearly and the maximum fracture stress declines gradually. When the content of AEO-AC-10-5 is less than 80%, the maximum broken stress goes down slightly, and when the content of AEO-AC-10-5 is more than 80%, it declines rapidly. The maximum broken stress of HA-gels containing sheer AEO-AC-13-5 is nearly quadruple that of containing pure AEO-AC-10-5 under the same situation. If the pure AEO-AC-10-5-AM gels were put into distilled water, they would be dissolved within two months, but the pure AEO-AC-13-5 gels still remain their shape over two months. From the Fig. 6, it can be observed that the range of the maximum broken stress changes from 10 kPa to 210 kPa. Therefore, it is a convenient method to adjust the mechanical properties of the gels by changing the ratio between straight carbon chain hydrophobic monomer and branched carbon chain hydrophobic monomer.
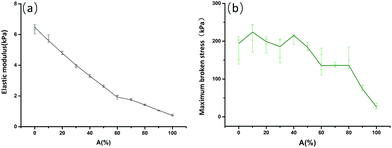 |
| | Fig. 6 Changes of tensile mechanical properties with altering ratio of AEO-AC-10-5 and AEO-AC-13-5 content. | |
Self-healing of HA-gels
All synthesized HA-gels possess self-healing capability. The hydrogel rod (Φ 6 mm × 4 cm) was cut into four separated parts, dyeing interval two of them with methylene blue for clearly observing. The cut parts were put onto the horizontal table closely without exerting external force. After 1 minute, they were self-healing quickly and could support their own weight, so we could pick up ‘the one rod’ by a tweezers (see Fig. 7a-4). When the cut two parts were put into a plastic tube, which was wrapped closely by plastic film, and then the plastic tube was put on a horizontal table, and the two parts were almost totally healed after 24 h (see Fig. 7b). HA-gels possess the capability of self-healing due to the dissociation and re-association process of the associated points.
 |
| | Fig. 7 Self-healing of HA-gels. | |
Experimental
Materials
Polyoxyethylene ether (AEO: CnH2n+1(OCH2CH2)5OH, n = 10, 13; CP grade) was purchased from Ningbo Lejia Chemical Co., China (the composition of AEO is listed in Table 1, provided by Lejia Chemical Co.). Sodium dodecyl sulphate (SDS, CP grade) was provided by Tianjin Guangfu Fine Chemical Research Institute. Acryloyl chloride (AC, CP grade) was purchased from Shanghai Haiqu Chemical Co., China. These reagents were used without further purification. Acrylamide (AM) and potassium persulfate (KPS) were provided by Tianjin Fuchen Chemical Reagent Factory, China, and recrystallized with distilled water before using. Trimethylamine (TEA) was provided by Tianjin Fuyu Chemical Reagent Factory, China. Dichloromethane CH2Cl2 was provided by Beijing Chemical works. These two agents were purified by distillation.
Table 1 The composition of AEO39
| AEO-n-5 |
Carbon chain length |
Content (%) |
| AEO-10-5 is isomeric decyl alcohol polyoxyethylene ether. Branched degree is 2.1. AEO-13-5 is a mixture of n-dodecanol polyoxyethylene ether and n-tetradecanol polyoxyethylene ether. |
| AEO-10-5a |
C9 |
4 |
| C10 |
89 |
| C11 |
3 |
| AEO-13-5b |
C12 |
50 |
| C14 |
50 |
Synthesis of hydrophobic monomers
AEO-AC monomers were synthesized as follows: 0.10 mol AEO, 0.12 mol TEA, 0.15 mol AC, and 160 mL CH2Cl2 were added to a three-neck flask with electromagnetic stirrer in an ice-salt-water bath. When a homogenous solution was achieved, 0.12 mol AC in 27 mL CH2Cl2 was added dropwise to the flask under stirring, and the temperature of water bath remained in the range of −5 to 0 °C. After adding the AC, the reaction was carried out for 5 h, and the CH2Cl2 was removed by rotary evaporation. To separate TEA hydrochloride that is the sediment of the reaction system, the appropriate amount of acetone was added into the mixture, and the upper clear liquid containing AEO-AC was distilled under reduced pressure to remove the diethyl ether. Finally, the residual TAC in the product was separated by centrifugation and the final product, AEO-AC was dried to constant weight in vacuum at 50 °C.
Synthesis of HA-gels
The HA-gels were synthesized by micellar copolymerization. The reaction system was generally consisted of water-soluble monomers, hydrophobic monomers, surfactants and water. As a typical example, AM (1.00 g), AEO-AC-10-5 (varied), AEO-AC-13-5 (varied), SDS (varied), and distilled water were added to a beaker, in which the total mass of the reaction solution was 10 g. The mixture was treated ultrasonically until a homogenous solution was achieved. Then, 0.50 mL of KPS solution (0.01 g mL−1) was added to above solution. Afterwards, the solution was put into a test tube and drove out dissolved oxygen with N2 for 10 min under normal pressure, and the reduced the pressure to dispel the air bubbles. Immediately, the test tube was sealed. After being placed at room temperature for 1 h, the test rube containing the reactant solution was heated to 50 °C in a water bath for 12 h, and then HA-gels with high mechanical properties were prepared. The compositions in initial reaction solution and nomenclature were listed in Table 2 for HA-gels.
Table 2 The compositions in initial reaction solution and nomenclature for HA-gels
| HA-gels |
AM (wt%) |
AEO-AC (mol%) |
SDS |
| X: the mole percentage of the hydrophobic monomer AEO-AC to hydrophilic monomer AM, X = 1, 2, 3. R: the mole percentage of the surfactant SDS to hydrophobic monomer AEO-AC, R = 0.5, 1.0, 2.0, 3.0, 4.0. A: the mole percentage of AEO-AC-10-5 in hydrophobic monomers (AEO-AC-10-5 + AEO-AC-13-5 = 100 mol%), A = 10, 20, 30, 40, 50, 60, 70, 80, 90, 100. |
| AEO-AC-n-5-AM (AEO-X%) |
10 |
Xa |
3 wt% |
| AEO-AC-n-5-AM (AEO-X%, SDS-R) |
10 |
X |
Rb |
| AEO-AC-mix-5-AM (10-5-A%)c |
10 |
2 |
3 wt% |
Characterization
Fourier transform infrared (FTIR) spectrum was recorded in the wave number range of 4000–500 cm−1 by using a Nicolet 360 FTIR spectrophotometer. The samples are AEO-AC, dried HA-gels, and homo-polymer of AM. Dried HA-gels and homo-polymer of AM were prepared by drying purified HA-gels and homo-polymer of AM in vacuum at 50 °C to constant weight.
Measurements of mechanical properties
The tensile stress–strain measurements were performed by using a universal tester at 25 °C (AG-I 1kN, Shimadzu, Japan). The tensile experiment was carried out at cross-head speed of 100 mm min−1, the cylindrical gels samples are 6 mm in diameter and the original length between top and foot clamps is 20 mm. The tensile relaxation measurements were performed by using a universal tester at 25 °C (AG-I 1kN, Shimadzu, Japan). In this study, stress relaxation tests were carried out after stretching the specimens (initial size of Φ 6 mm × 2 cm length) to a certain elongation (500%) for 2 h.
Measurements of swelling behaviour
Swelling experiments were performed by immersing as-prepared HA-gels (initial size of Φ 6 mm × 5 mm length) in a large excess of distilled water at room temperature and distilled water was replaced daily. In each measurement, the samples were removed from water and weighed after removing excess distilled water from their surface, and then these samples were dried to constant weight in oven at 50 °C. The swelling degree was expressed by the ratio of the weight of the swollen hydrogel to its corresponding dried gel weight, and the gel fraction was expressed by the ratio of the dried gel weight after a certain swelling time to its theoretical dried gel weight. Here, the theoretical dried gel weight means theoretical solid weight based on the experimental recipe.
The theoretical solid content of gels (S%), swelling degree (Sr) and the remaining gel fraction (GF) were calculated by equations below:
| |
 | (1) |
| |
 | (2) |
| |
 | (3) |
Where
Wt = the weight of swollen hydrogel sample at regular time interval
t;
Wd = the weight of corresponding dry gel;
Wo = the ordinary weight of hydrogel; five unswelled gels in each group were dried directly after been cut,
WA = the total weight of these five unswelled gels after drying,
WB = the total original weight of these gels.
Conclusions
We have successfully synthesized a novel kind of hydrophobic association hydrogels via micellar copolymerization. The prepared two series of HA-gels exhibit differently in mechanical properties, stress relaxation behaviour and swelling behaviour. As AEO-AC-13-5 has longer and sheer straight carbon chain, the associated point in AEO-AC-13-5-AM gels is stronger. Under exegetic action, the hydrophobic segment in AEO-AC-13-5-AM gels is hardly extracted from the hydrophobic associated domain. Meanwhile, AEO-AC-10-5-AM gels have shorter and branched carbon chain which forms weaker hydrophobic associated points. The hydrophobic segment in AEO-AC-10-5-AM is easily extracted from the hydrophobic associated domain. Thus compared to AEO-AC-10-5-AM gels, AEO-AC-13-5-AM gels have stronger mechanical properties, longer stress relaxation time, lower swelling degree. Some AEO-AC-13-5-AM gels with low content of SDS even can keep their shapes in water nearly half a year kin to permanent gels. In contrast, AEO-AC-10-5-AM gels with high content of SDS dissolved in water quickly within 4 days. We can control the strength of hydrogels by varying the ratio of AEO-AC-10-5 to AEO-AC-13-5 monomers and adjusting the content of surfactant SDS. It is a simple and convenient method to adjust the mechanical properties of HA-gels by tuning the ratio of hydrophobic monomer AEO-AC-13-5 and AEO-AC-10-5. All the prepared HA-gels are self-healing.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21174053).
Notes and references
- M. L. Gou, X. Y. Li, M. Dai, C. Y. Gong, X. H. Wang, Y. Xie, H. X. Deng, L. J. Chen, X. Zhao, Z. Y. Qian and Y. Q. Wei, Int. J. Pharm., 2008, 359, 228 CrossRef CAS PubMed.
- C. V. Duffy, L. David and T. Crouzier, Acta Biomater., 2015, 20, 51 CrossRef CAS PubMed.
- A. O. Abioye, S. Issah and A. T. K. Mustapha, Int. J. Pharm., 2015, 490, 112 CrossRef CAS PubMed.
- M. F. Refojo, J. Polym. Sci., Part A-1: Polym. Chem., 1967, 5, 3103 CrossRef CAS PubMed.
- J. I. Jay, K. Langheinrich, M. C. Hanson, A. Mahalingam and P. F. Kiser, Soft Matter, 2011, 7, 5826 RSC.
- S. M. R. K. Mofrad, F. Naeimpoo, P. Hejaz and A. Nematollahzadeh, J. Appl. Polym. Sci., 2014, 5, 132 Search PubMed.
- P. A. Vivero, E. F. Gabriel, C. A. Lorenzo and A. Concheiro, J. Pharm. Sci., 2007, 96, 802 CrossRef PubMed.
- M. Zhong, X. Y. Liu, F. K. Shi, L. Q. Zhang, X. P. Wang, A. G. Cheetham, H. G. Cui and X. M. Xie, Soft Matter, 2015, 11, 4235 RSC.
- F. K. Shi, X. P. Wang, R. H. Guo, M. Zhong and X. M. Xie, J. Mater. Chem. B, 2015, 3, 1187 RSC.
- J. Wang, Y. S. Xu, Y. M. Wang, J. J. Liu, L. L. Xu, H. T. Nguyen, D. T. Pham, S. F. Lincolnc and X. Gu, RSC Adv., 2015, 5, 46067 RSC.
- A. Sandier, W. Brown, H. Mays and C. Amiel, Langmuir, 2000, 16, 1634 CrossRef CAS.
- X. L. Zhang, X. L. Chu, L. Wang, H. M. Wang, G. L. Liang, L. X. Zhang, J. F. Long and Z. M. Yang, Angew. Chem., 2012, 124, 4464 CrossRef.
- N. Ruocco, H. Frielinghaus, G. Vitiello, G. D'Errico, L. G. Leal, D. Richter, O. Ortona and L. Paduano, J. Colloid Interface Sci., 2015, 452, 160 CrossRef CAS PubMed.
- O. Ortona, G. D'Errico, G. Mangiapia and D. Ciccarelli, Carbohydr. Polym., 2008, 74, 16 CrossRef CAS.
- Q. C. Li, D. G. Barrett, P. B. Messersmith and N. H. Andersen, ACS Nano, 2015, 10, 1317 CrossRef PubMed.
- N. Holten-Andersen, A. Jaishankar, M. J. Harrington, D. E. Fullenkamp, G. Dimarco, L. He, G. H. McKinley, P. B. Messersmith and K. Y. C. Lee, J. Mater. Chem. B, 2014, 2, 2467 RSC.
- D. C. Tuncaboylu, A. Argun, M. Sahin, M. Sari and O. Okay, Polymer, 2012, 53, 5513 CrossRef CAS.
- M. A. Haque, T. Kurokawa and J. P. Gong, Polymer, 2012, 53, 1805 CrossRef CAS.
- Z. B. Zhao, S. S. An, H. J. Xie and Y. Jiang, Chin. J. Polym. Sci., 2015, 33, 173 CrossRef CAS.
- M. Zhong, F. K. Shi, Y. T. Liu, X. Y. Liu and X. M. Xie, Chin. Chem. Lett., 2016, 27, 312 CrossRef CAS.
- S. S. Zhang, D. J. Alvarez, M. V. Sofroniew and T. J. Deming, Biomacromolecules, 2015, 16, 1331 CrossRef CAS PubMed.
- S. Hocine and M. H. Li, Soft Matter, 2013, 9, 5839 RSC.
- K. A. Watkins and R. Chen, Int. J. Pharm., 2015, 478, 496 CrossRef CAS PubMed.
- H. F. Jiang, S. Y. Qin, H. Dong, Q. Lei, X. Su, R. X. Zhuo and Z. L. Zhong, Soft Mater., 2015, 11, 6029 RSC.
- W. S. Cai, C. L. Feng, X. Y. Ma, M. N. Chen and J. Y. Liu, Electrochim. Acta, 2015, 169, 424 CrossRef CAS.
- D. Morales, E. Palleau, M. D. Dickey and O. D. Velev, Soft Mater., 2014, 10, 1337 RSC.
- C. Liu, X. L. Liu, J. F. Yu, G. Gao and F. Q. Liu, J. Appl. Polym. Sci., 2014, 10, 1002 Search PubMed.
- G. Q. Jiang, C. Liu, X. L. Liu, G. H. Zhang, M. Yang, Q. R. Chen and F. Q. Liu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2010, 47, 335 CrossRef CAS.
- L. Zha, J. Hu, C. Wang, S. Fu, A. Elaissari and Y. Zhang, Colloid Polym. Sci., 2002, 280, 1 CAS.
- Y. Y. Liu, W. Q. Liu, W. X. Chen, L. Sun and G. B. Zhang, Polymer, 2007, 48, 2665 CrossRef CAS.
- Q. R. Chen, C. Liu, G. Q. Jiang, X. L. Liu, M. Yang, D. Zhang and F. Q. Liu, Acta Polym. Sin., 2010, 6, 797 CrossRef.
- B. Yang, D. Xu, X. L. Wu, Z. J. Li, L. C. Lei and X. W. Zhang, J. Ind. Eng. Chem., 2015, 25, 67 CrossRef CAS.
- R. Anzai and Y. Murakami, Colloids Surf., B, 2015, 127, 292 CrossRef CAS PubMed.
- G. Q. Jiang, C. Liu, X. L. Liu, G. H. Zhang, M. Yang, Q. R. Chen and F. Q. Liu, Macromol. Mater. Eng., 2010, 294, 815 Search PubMed.
- G. Q. Jiang, C. Liu, X. L. Liu, G. H. Zhang, M. Yang, Q. R. Chen and F. Q. Liu, Polymer, 2010, 51, 1507 CrossRef CAS.
- Z. Liang, T. T. Gao, J. N. Xu, Z. Y. Li, X. L. Liu and F. Q. Liu, Chem. Res. Chin. Univ., 2015, 2, 5122 Search PubMed.
- C. Liu, J. F. Yu, X. L. Liu, Z. Y. Li, G. Gao and F. Q. Liu, J. Mater. Sci., 2013, 48, 774 CrossRef CAS.
- M. Yang, C. Liu, Z. Y. Li, G. Gao and F. Q. Liu, Macromolecules, 2010, 43, 10645 CrossRef CAS.
- L. Chen, Master Thesis in China National Knowledge Internet, Jiangnan University, Wuxi, 2012, vol. 2, p. 11.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O; the peak at 1640 cm−1 corresponding to stretching vibration of C
O; the peak at 1640 cm−1 corresponding to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; the peak at 1410 cm−1 corresponding to the shear-vibration of
C; the peak at 1410 cm−1 corresponding to the shear-vibration of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and the peak at 1195 cm−1 corresponding to the asymmetric stretching vibration of C–O–C. All these evidences demonstrate that AEO-AC was successfully synthesized.
CH2 and the peak at 1195 cm−1 corresponding to the asymmetric stretching vibration of C–O–C. All these evidences demonstrate that AEO-AC was successfully synthesized.











