Biocompatible natural sugar-based surfactant assisted oxidation of citric acid by MnO4− in absence and presence of SDS†
Abstract
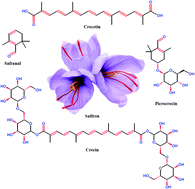
Crocin, a natural carotenoid with antioxidant properties, was used in the present investigation as a surfactant in the citric acid–MnO4− redox system for the first time. A conventional UV-Visible spectroscopic technique was used to determine the reaction rate with and without crocin. The reaction follows first-order kinetics with respect to [citric acid] under pseudo-first conditions. Crocin was found to catalyze the redox reaction, which was rationalized in terms of the solubilization and/or incorporation of reactants into the crocin aggregates. The micellar catalysis was explained using Menger–Portony pseudo-phase model modified by Bunton et al. The various parameters associated with the micellar catalysis and activation parameters were determined and discussed. Sodium dodecyl sulphate (SDS) inhibits citric acid oxidation. A mixture of non-ionic and anionic surfactant (crocin + SDS) also shows the inhibitory effect rather than catalytic effect. In the mix micellization, the SDS characters and electrostatic repulsion, dominate over the solubilization of reactants in to the Stern layer. On the basis of the observed results, probable mechanisms and reaction sites were proposed.

 Please wait while we load your content...
Please wait while we load your content...