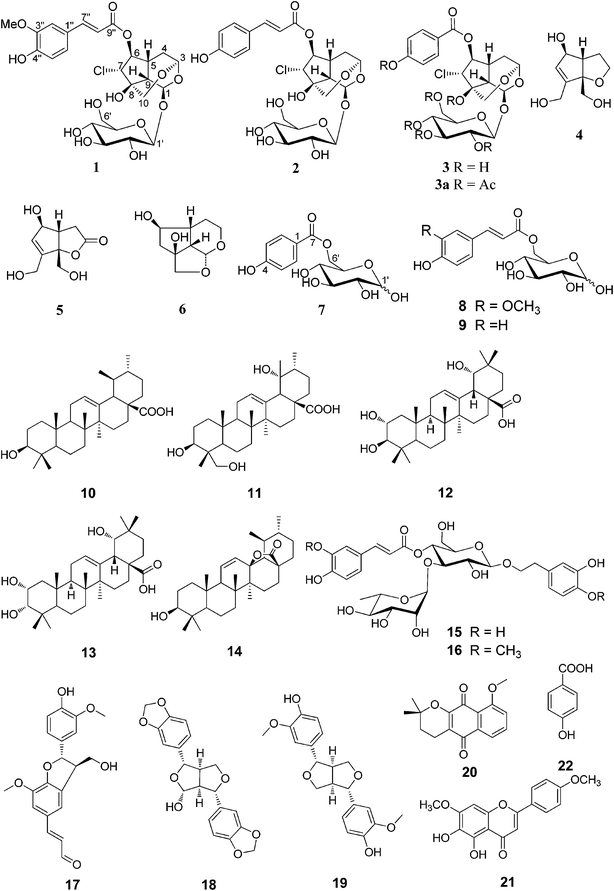
Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities†
Hao-Yu Tanga,
Meng-Meng Baia,
Jun-Mian Tiana,
Gennaro Pescitellib,
Trpimir Ivšićbc,
Xiao-Hua Huang*a,
Hyunwoo Leed,
Ya Nan Sond,
Jang Hoon Kimd,
Young Ho Kimd and
Jin-Ming Gao*a
aShaanxi Key Laboratory of Natural Products & Chemical Biology, College of Science, Northwest A&F University, Yangling 712100, P. R. China. E-mail: jinminggao@nwsuaf.edu.cn; x.h.huang@163.com; Tel: +86-29-87092515
bDipartimento di Chimica e Chimica Industriale, Università di Pisa, via Moruzzi 3, I-56124 Pisa, Italy
cDivision of Organic Chemistry and Biochemistry, Ruđer Bošković Institute, 10000 Zagreb, Croatia
dCollege of Pharmacy, Chungnam National University, Daejeon 305-764, Republic of Korea
First published on 19th April 2016
Abstract
Two new chlorinated iridoids, named bungosides A (1) and B (2), were isolated from the seeds of Catalpa bungei (family Bignoniaceae). Their structures were elucidated on the basis of NMR analysis. Twenty known compounds (3–22) were also characterized, including in one case (6-O-p-hydroxybenzoylglutinoside, 3) the assignment of the absolute configuration by employing electronic circular dichroism (CD) and time-dependent density functional theory (TDDFT) calculations. Compounds 1–3 were unusual cage-like iridoids with an oxygen bridge between C-3 and C-10. Among the isolates, ursolic acid lactone (14) was the most active soluble epoxide hydrolase (sEH) inhibitor with an IC50 19.1 ± 0.8 μM. In addition, D-pinoresinol (19) and ladanein (21) displayed selectively inhibitory effects on butyrylcholinesterase (BChE) with IC50 of 31.5 ± 0.4 μM and 33.0 ± 2.3 μM, respectively, but not acetylcholinesterase (AChE) activity, and only compound 18 suppressed NF-κB activity in HepG2 cells (IC50 21.53 ± 2.37 μM).
Introduction
Soluble epoxide hydrolase (sEH) is an important bifunctional enzyme with potent biological activities on vascular and renal systems. It can hydrolyze epoxyeicosatrienoic acids (EETs), metabolites of arachidonic acid generated by epoxygenase CYP enzymes, into dihydroxyeicosatrienoic acids (DHETs).1 The former produce significant biological effects in vascular and renal systems while the latter don't show much beneficial function. EETs are endothelium-derived hyperpolarizing factors (EDHFs) which act as regulators of vascular function.2 The cardiovascular effects of EETs include vasodilation, antimigratory actions on vascular smooth muscle cells and anti-inflammatory action.1 Therefore, the inhibition of sEH becomes an effective pathway to treat cardiovascular disease. Alzheimer's disease (AD) is one of the most common neurodegenerative diseases in elderly population. Reduced synthesis of the neurotransmitter acetylcholine is thought to be a main cause of AD.3 Therefore, cholinesterase inhibitors became an important cognitive enhancing treatment based on the facts that they could increase the content of acetylcholine at cholinergic synapses. Both acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) participate in hydrolyzing brain acetylcholine in different manners.4 AChE and BChE have thus been the main targets to prevent AD.Inflammation contributes to the pathogenesis of cancer, cardiovascular diseases, and type-2 diabetes mellitus.5 The nuclear factor kappaB (NF-κB) is a key regulator of many pro-inflammatory pathways.5 NF-κB normally resides in the cytoplasm, where it is retained by association with the endogenous inhibitor of kappa B (IκB) protein.6 However, when activated by inflammatory stimuli such as tumor necrosis factor alpha (TNF-α), injury, or other stress-related stimuli, NF-κB is released, translocates to the nucleus and binds to DNA resulting in the transcription of proinflammatory target genes.7 Therefore, inhibitors targeting NF-κB signaling are considered as potential candidates for both prevention and therapy of inflammation.
The Catalpa plants of Bignoniaceae family include about 13 species in the world and are mainly distributed in America and East Asia. Previous phytochemical investigation indicated that Catalpa plants contain iridoids and naphthoquinones as the main constituents with various pharmacological properties such as antifungal, cytotoxic, antitumor, and antiplasmodial activities.8–11 Catalpa bungei C. A. Mey is widely distributed in China, and the fruits of the tree have been used in folk medicine for edema, nephritis, cystitis, eczema, leprosy and gastric cancer.12 However, few secondary metabolites from the seeds of this plant have been reported.
In continuation of our investigation on the bioactive constituents from traditional Chinese medicines in the Qinba Mountains,13–16 we carried out chemical investigations on the seeds of C. bungei and evaluated their sEH, AChE and BChE inhibitory activities. In this study, two new compounds (1, 2), along with 20 known natural compounds that belong to various groups of iridoids, triterpenoids, phenylethanoid glycosides, and lignans, were isolated from the seeds of C. bungei. Their inhibitions of sEH, AChE and BChE were also assessed. Herein, we report the isolation and structural elucidation of the new compounds (1, 2), as well as enzymatic inhibitory activity of these compounds. The present study could lay a foundation for the medicinal development of C. bungei.
Results and discussion
The EtOAc-soluble fraction of a 75% EtOH extract of seeds of C. bungei, was chromatographed by means of different chromatographic techniques using normal-phase silica gel, reversed-phase C18 gel, Sephadex LH-20 and RP-HPLC to afford three new (1 and 2) and 20 known compounds (Fig. 1).The identification of the known compounds, des-p-hydroxybenzoyl kisasagenol B (4),11 rehmaglutin C (5),17 des-p-hydroxybenzoyl-3-deoxycatalpin (6),18 6-O-(p-hydroxybenzoyl)-D-glucopyranose (7),19 6-O-[(E)-feruloyl]-D-glucopyranose (8),20 6-O-p-coumaroyl-D-glucopyranose (9),21 ursolic acid (10),22 rotundic acid (11),23 arjunic acid (12),24 2α,3α,19α-trihydroxy-12-oleanen-28-oic acid (13),25 ursolic acid lactone (14),26 martynoside (15),27 verbascoside (kusaginin or acteoside, 16),28 balanophonin (17),29 9α-hydroxysesamin (18),30 D-pinoresinol (19),31 9-methoxy-α-lapachone (20),32 ladanein (21),33 and p-hydroxybenzoic acid (22),34 was confirmed by comparing their physical and spectroscopic data with those reported in the literature. The known compounds included various types of iridoids, triterpenoids, phenylethanoid glycosides, and lignans. To the best of our knowledge, compounds 1–22 (except 3) were isolated from C. bungei for the first time, of which 7–9, 11–14, and 17–19, in particular the occurrence of ursane and oleanane triterpenes, were first isolated from the genus of Catalpa.
Compound 3 was obtained as a white powder. The molecular formula was established as C22H27ClO12 by HR-ESI-MS at m/z 541.1081 [M + Na]+, indicating nine degrees of unsaturation. Its IR spectrum indicated the presence of hydroxy (3400 cm−1), conjugated carbonyl ester (1693 cm−1), and aromatic (1607 and 1514 cm−1) functionalities in the molecule. The 13C NMR and DEPT data (Table 1) exhibited 22 signals, including three methylenes, fifteen methines, and four quaternary carbons. The 1H and 13C NMR spectroscopic data suggested the presence of two acetal groups (δH 5.70, δC 93.1 and δH 5.36, δC 95.9), one tertiary hydroxyl group (δC 79.9), one oxymethylene (δH 4.09 and 3.72, δC 62.1), an aromatic unit, and a β-glucopyranosyl moiety. These assignments were further confirmed by the HMBC correlations forming the cyclopentanopyrane ring skeleton, a p-hydroxybenzoyl and a β-glucopyranosyl residue. The 1H NMR spectrum (Table 1) showed the presence of a doublet signal at δH 5.70 (J = 2.1 Hz, 1H) that is a characteristic signal for H-1 of an iridoid.35 The 13C signals at δC 98.9, 74.2, 78.1, 71.6, 78.1, and 62.7 in the 13C NMR spectrum were characteristic for a β-D-glucopyranosyl residue. The β-configuration for the glucose was supported from the large coupling constant (J = 8.0 Hz) for the anomeric doublet signal at δH 4.73 (H-1′). HMBC correlations of δH 5.70 (H-1) and δC 98.9 (C-1′), and δH 4.73 (H-1′) and δC 93.1 (C-1) indicated that the glucopyranosyl unit was attached to C-1 of the aglycone. The chemical shift of C-7 at δC 70.4 indicated that the Cl atom was located at C-7.36,37 Detailed interpretation of the 1H and 13C NMR spectral data (Table 1) indicated that 3 contains a p-hydroxybenzoyl group [δH 7.92 (d, J = 8.8 Hz, H-2′′,6′′), 6.87 (d, J = 8.8 Hz, H-3′′,5′′); δC 168.6 (C-7′′), 164.3 (C-4′′), 121.4 (C-1′′)]. The attachment of the p-hydroxybenzoyl unit on C-6 (δC 87.9) of the aglycone was established from the HMBC correlation between H-6 at δH 5.13 and the ester carbonyl carbon (δC 168.6) of the p-hydroxybenzoyl group (Fig. 2).
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (multi. J in Hz) | δC | δH (multi. J in Hz) | δC | δH (multi. J in Hz) | |
| 1 | 93.1 d | 5.70 d, 2.1 | 93.1 d | 5.64 d, 2.0 | 93.1 d | 5.70 d, 2.1 |
| 3 | 95.9 d | 5.35 d, 3.0 | 95.9 d | 5.30 d, 2.9 | 95.9 d | 5.36 d, 3.0 |
| 4 | 34.4 t | 2.49 dd, 13.7, 8.3; 2.13 dd, 13.7, 3.0 | 34.4 t | 2.43 dd, 13.6, 8.5; 2.07 dd, 13.6, 2.9 | 34.4 t | 2.49 dd, 13.5, 8.5; 2.16 dd, 13.5, 3.0 |
| 5 | 34.8 d | 2.40, m | 34.7 d | 2.34 m | 34.7 d | 2.43, m |
| 6 | 87.7 d | 5.05 dd, 8.3, 2.7 | 87.7 d | 4.99 dd, 8.3, 2.7 | 87.9 d | 5.13 dd, 8.3, 2.7 |
| 7 | 70.4 d | 4.46 d, 8.3 | 70.4 d | 4.41 d, 8.1 Hz | 70.4 d | 4.54 dd, 8.3, 0.9 |
| 8 | 79.9 s | 79.9 s | 79.9 s | |||
| 9 | 48.3 d | 2.65 d, 9.9 | 48.3 d | 2.59 d, 9.9 | 48.3 d | 2.67 d, 9.8 |
| 10 | 62.1 t | 4.08 d, 11.9; 3.72 d, 11.9 | 62.1 t | 4.02 d, 11.8; 3.65 m | 62.1 t | 4.09 dd, 12.2, 0.8; 3.72 d, 12.2 |
| 1′ | 99.0 d | 4.73 d, 8.0 | 98.9 d | 4.67 d, 8.3 | 98.9 d | 4.73 d, 8.0 |
| 2′ | 74.7 d | 3.19 dd, 9.1, 8.0 | 74.7 d | 3.14 t, 8.3 | 74.2 d | 3.18 dd, 9.0, 8.0 |
| 3′ | 78.2 d | 3.41, m | 78.1 d | 3.35, m | 78.1 d | 3.40, m |
| 4′ | 71.6 d | 3.31, m | 71.6 d | 3.26, m | 71.6 d | 3.31, m |
| 5′ | 78.1 d | 3.31, m | 78.1 d | 3.28, m | 78.1 d | 3.30, m |
| 6′ | 62.7 t | 3.70, m; 3.90, m | 62.7 t | 3.85, m; 3.65, m | 62.7 t | 3.70, m; 3.91, m |
| 1′′ | 127.6 s | 127.0 s | 121.4 s | |||
| 2′′ | 111.9 s | 7.25 d, 1.8 | 131.3 d | 7.46 d, 8.6 | 132.9 d | 7.92 d, 8.8 |
| 3′′ | 149.4 s | 116.9 d | 6.79 d, 8.6 | 116.4 d | 6.87 d, 8.8 | |
| 4′′ | 150.8 s | 161.5 s | 164.3 s | |||
| 5′′ | 116.5 d | 6.85 d, 8.2 | 116.9d | 6.79 d, 8.6 | 116.4 d | 6.87 d, 8.8 |
| 6′′ | 124.3 d | 7.13 dd, 8.2, 1.8 | 131.3 d | 7.46 d, 8.6 | 132.9 d | 7.92 d, 8.8 |
| 7′′ | 147.5 d | 7.68 d, 15.9 | 147.2 d | 7.62 d, 15.8 | 168.6 s | |
| 8′′ | 114.9 d | 6.45 d, 15.9 | 114.5 d | 6.36 d, 15.9 | ||
| 9′′ | 168.8 s | 168.9 s | ||||
| OCH3 | 56.5 q | 3.93, s | ||||
The relative stereochemistry at C-1, C-5, and C-9 was determined to be the same as found in other iridoid glucosides based on the small coupling of J1,9 (2.1 Hz) and the large coupling of J5,9 (9.8 Hz). The configuration of the 8-OH was confirmed to be β from the C-9 chemical shift (δC 48.3), which is a characteristic of assignment of the configuration of the 8-OH.38 This β-configuration causes the deshielding of C-9 in comparison to its α counterpart in the following pairs of C-8 isomers, 10-des-cinnamoylglobularimin and 10-des-cinnamoylglobularinin,39 and gardenoside and monotropein methyl ester.38 The configuration of the H-3 proton was determined to be β on the basis of the formation of the intramolecular acetal of C-3 with the C-10 OH.40 As a result, compound 3 was elucidated as a rigid tricyclic iridoid. Similar iridoids possessing a cage-like skeleton have been isolated from Picrorhiza kurroa,41 Catalpa bignonioides,42 and Rehmannia glutinosa43 and also obtained through bromination and dehydration of the catalposide extracted from the roots of Picrorhiza kurroa.44
Compound 3 is a derivative of glutinoside, which was isolated previously from the dried root of Rehmannia glutinosa.45 It had not been isolated previously as a pure natural form but as a semi-synthetic hexaacetate, obtained through acetylation of crude extract of Catalpa species.11 Here, the NMR data of 3 are reported for the first time. The structure of this compound was confirmed by detailed analysis of the 2D-NMR data (HSQC, HMBC, 1H–1H COSY, and NOESY spectra) (Fig. 2). On the basis of the above analysis, the structure of 3 was determined to be 6-O-p-hydroxybenzoylglutinoside. In order to further determine the structure of 3, we prepare its hexaacetate (3a), whose NMR data are in good agreement with published data.11 Since the absolute configuration of the hexaacetate of 3 was not directly determined in the original paper,11 we decided to assign it independently by means of electronic circular dichroism (CD) spectroscopy. The experimental UV absorption and CD spectrum of (−)-3 in acetonitrile are reported in Fig. 3. The UV consists of two main bands mainly associated with the electronic transitions of the p-hydroxy benzoate chromophore. Correspondingly, the CD spectrum shows three major bands above 220 nm and a positive tail at shorter wavelengths. To simulate the CD spectrum by TDDFT calculations,46 we first generated a set of structures reproducing low-energy conformers in solution, using a well-established procedure based on a Monte Carlo conformational search followed by geometry optimizations with DFT method. The resulting set of conformations was composed by two main families, one devoid of any intramolecular hydrogen bond, and one showing consistently an intramolecular hydrogen bond between glucose 6′-OH and the ester carbonyl group. Such a hydrogen bond leads to the formation of a 14-membered cycle which is quite disfavored by entropic factors, and it is likely to be a computational artifact.47 In fact, the NOESY spectrum of 3 did not show any cross-peak expected for such a cyclic structure, e.g. between H-2′′ and CH2-6′. Therefore, we neglected these hydrogen-bonded conformers from our set; however we also checked that the inclusion of hydrogen-bonded conformers in TDDFT calculations would not affect the final assignment (results not shown). As a consequence, we obtained a restricted set of low-energy conformers including 9 structures with relative energies within 1.8 kcal mol−1 and populations >1% at room temperature; the lowest-energy conformer is shown in the inset in Fig. 3. All 9 conformers share the same conformation of the polycyclic skeleton, which seems to be very rigid, and also the same conformation of the p-hydroxy benzoate group, defining a dihedral angle (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )C–O–C-6–H-6 around −40°. The 9 structures differ only in the flip of 4′′-OH and in the rotamerism of the glucose OH groups. Accordingly, the CD spectra calculated with TDDFT method at B3LYP/TZVP level for the whole conformational set were similar to each other. The Boltzmann-weighted average of the spectra is shown in Fig. 3. The CD spectrum calculated for enantiomer (1S,3R,5R,6S,7R,8S,9S)-3 is in very good agreement with the experimental CD spectrum measured for (−)-3. Therefore, we may assign 6-O-p-hydroxybenzoyl glutinoside the absolute configuration (−)-(1S,3R,5R,6S,7R,8S,9S). Not surprisingly, this is the same configuration reported for the analogs of glutinoside,45 6-O-p-hydroxybenzoyl asystasioside E11 and piscroside A,48 all of which are ultimately related to rehmaglutin D.49
)C–O–C-6–H-6 around −40°. The 9 structures differ only in the flip of 4′′-OH and in the rotamerism of the glucose OH groups. Accordingly, the CD spectra calculated with TDDFT method at B3LYP/TZVP level for the whole conformational set were similar to each other. The Boltzmann-weighted average of the spectra is shown in Fig. 3. The CD spectrum calculated for enantiomer (1S,3R,5R,6S,7R,8S,9S)-3 is in very good agreement with the experimental CD spectrum measured for (−)-3. Therefore, we may assign 6-O-p-hydroxybenzoyl glutinoside the absolute configuration (−)-(1S,3R,5R,6S,7R,8S,9S). Not surprisingly, this is the same configuration reported for the analogs of glutinoside,45 6-O-p-hydroxybenzoyl asystasioside E11 and piscroside A,48 all of which are ultimately related to rehmaglutin D.49
Compound 1 was obtained as a white powder. The molecular formula C25H31ClO13 was established by HR-ESI-MS at m/z 597.1343 [M + Na]+, requiring 10 degrees of unsaturation. Its IR spectrum indicated the presence of hydroxy (3393 cm−1) and α,β-unsaturated ester carbonyl (1697 cm−1) groups, and an aromatic moiety (1598, 1516 cm−1) in the molecule. The 13C NMR and DEPT data (Table 1) showed the presence of one methyl, three methylenes, sixteen methines, and five quaternary carbons. The presence of a sugar moiety was evidenced by a set of signals observed in the 1H NMR spectrum between δH 4.73 and 3.19, and signals observed in the 13C NMR spectrum (Table 1) at δC 62.7, 71.6, 74.7, 78.1, 78.2, and 99.0. The coupling constant of the anomeric proton at δH 4.73 (d, J = 8.0 Hz, H-1′) indicated the β-configuration of the glucopyranose. Acid hydrolysis afforded D-glucose based on GC analysis.
Detailed interpretation of the 1H and 13C NMR spectral data (Table 1) indicated that the chemical structure of compound 1 was very similar to those of 6-O-p-hydroxybenzoylglutinoside (3), except for the presence of an (E)-p-feruloyl group [δH 7.25 (d, J = 1.8 Hz, H-2′′), 7.13 (dd, J = 8.2, 1.9 Hz, H-6′′), 6.85 (d, J = 8.2 Hz, H-5′′), 7.68 (d, J = 15.9 Hz, H-7′′), 6.45 (d, J = 15.9 Hz, H-8′′); δC 168.8 (C-9′′), 127.6 (C-1′′)] in 1. The location of the feruloyl group at C-6 (δC 87.7) was supported by the correlation of H-6 (δH 5.05, dd, J = 8.3, 2.7 Hz) to the ester carbonyl at C-9′′ (δC 168.8) in the HMBC spectrum (Fig. 2). The HMBC correlations of the H-1 acetal signal at δH 5.70 (d, J = 2.1 Hz) to the C-1′ anomeric carbon at δC 99.0 and the C-3 acetal carbon at δC 95.9 indicated that the glucose residue was attached to C-1 of the aglycon, which was further linked to C-3 through an ether linkage. In addition, on the basis of the downfield shift of C-10 (δC 62.1) and HMBC correlation from H2-10 (δH 4.08, 3.72) to C-3 (δC 95.9), the acetal carbon C-3 was determined to be linked to C-10 through an oxo bridge. The methine carbon at δC 70.4 was assigned to C-7, attached to one chlorine atom,36,37 which was confirmed from the HMBC correlation of H2-10 to C-7 and from HR-ESI-MS data.
The relative configuration of 1 was confirmed by a NOESY experiment (Fig. 2) as well as from biosynthetic considerations. According to molecular modeling of this compound, the oxo bridge from C-3 to C-10 could only be α-oriented, and the 8-OH and H-9 could only be β-oriented. In the NOESY spectrum, the correlations between H-7 and H-5β, H-7 and H-9β, and H-6 and H-1α indicated that H-7 and H-6 were in β- and α-orientation, respectively, thereby establishing the stereochemistry of the Cl and the feruloyl group as α and β, respectively. In addition, the chemical-shift as well as coupling-constant values of the iridoid moiety of 1 (Table 1) were very similar to those reported for compound 3, suggesting that 1 possesses the same configuration (−)-(1S,3R,5R,6S,7R,8S,9S) as 3. The structure of this compound was confirmed by detailed analysis of the 2D-NMR data including its HSQC, HMBC, 1H–1H COSY, and NOESY spectra (Fig. S5–S8 and Table S1 in the ESI†). Consequently, the structure of 1 was determined to be 6-O-feruloylglutinoside, named bungoside A.
Compound 2 was assigned a molecular formula of C24H29ClO12, as established by the HR-ESI-MS at m/z 567.1243 [M + Na]+. The IR, UV, and NMR spectroscopic data (Table 1) of 2 closely resembled those of 1, except that the C-6 substituent group in 2 is a (E)-p-coumaroyl group [δH 7.46 (2H, d, J = 8.6 Hz, H-2′′,6′′), 6.79 (2H, d, J = 8.6 Hz, H-3′′,5′′), 7.62 (1H, d, J = 15.9 Hz, H-7′′), 6.36 (1H, d, J = 15.9 Hz, H-8′′); δC 168.9 (C-9′′), 127.0 (C-1′′)] instead of an (E)-feruloyl group. This was supported by the correlation of H-6 at δH 4.99 (dd, J = 8.3, 2.7 Hz) to the ester carbonyl at C-9′′ (δC 168.9) in the HMBC spectrum. The structure of this compound was confirmed by detailed analysis of the 2D-NMR data including its HSQC, HMBC, 1H–1H COSY, and NOESY spectra (Fig. S14–S17 and Table S2 in the ESI†). ROESY correlations suggested the same relative configuration as 1 and 3. Similarly, compound 2 was suggested to possess the same absolute configuration (−)-(1S,3R,5R,6S,7R,8S,9S) due to the same sign of specific rotation as 3 and 1 as well as to their same biogenetic relationships. Consequently, the structure of 2 was established to be (−)-(1S,3R,5R,6S,7R,8S,9S)-6-O-(E)-p-coumaroylglutinoside, named bungoside B.
The compounds 1–22 (except 9) were tested for their inhibitory effects on soluble epoxide hydrolase,16 acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) at 100 μM (Table S4†). Compounds 1, 8, 10, 14, 15, 17 and 20 showed inhibitory rate over 50% on sEH. Their IC50 values were determined in the following work (Table 2). Among the seven compounds tested, 14 showed the most active inhibitory activity on sEH with an IC50 value of 19.1 ± 0.8 μM; other compounds exhibited weak activity with IC50 values of 30–50 μM. In addition, kinetic curve of 14 on sEH showed it inhibits sEH by a mixed mode with inhibition constant Ki value 7.9 ± 1.7 μM (Fig. 4). Compounds 19 and 21 were found to display satisfactory inhibitory effects on AChE and BChE. Then only components 19 and 21 were tested their IC50 values on the two enzymes, and the results are observed in Table 2. Compounds 19 and 21 displayed selectively moderate inhibitory effects on butyrylcholinesterase (BChE) with IC50 values of 31.5 ± 0.4 μM and 33.0 ± 2.3 μM, respectively, but not acetylcholinesterase (AChE) activity. Moreover, kinetic curve of 19 and 21 on BChE (Table 2) showed they inhibited this enzyme by a mixed mode with Ki values of 7.8 ± 0.3 and 11.3 ± 2.9 μM, respectively (Fig. 5). To the best of our knowledge, this is the first report on 14 as sEH inhibitor as well as on the inhibition of 21 on BChE.
| compda | sEH inhibitory activity IC50a,c (μM) | AChE inhibitory activity IC50c,d (μM) | BChE inhibitory activity IC50c,d (μM) | NF-κB inhibitory activity IC50c,d (μM) |
|---|---|---|---|---|
| a All compounds were examined in a set of experiments three times.b Positive control.c Statistical significance was determined by one-way analysis of variance followed by Dunnett's multiple comparison test, P < 0.05 versus control.d Data were expressed as mean ± SD of at least three experiments performed in triplicate.e NT not tested. | ||||
| 1 | 50.4 ± 2.0 | NTe | NT | ND |
| 2 | NT | NT | NT | >100 |
| 3 | NT | NT | NT | 52.78 ± 2.26 |
| 4 | NT | NT | NT | >100 |
| 5 | NT | NT | NT | ND |
| 6 | NT | NT | NT | ND |
| 7 | NT | NT | NT | ND |
| 8 | 44.6 ± 3.4 | NT | NT | >100 |
| 9 | — | NT | — | — |
| 10 | 36.6 ± 7.7 | NT | NT | 33.93 ± 2.53 |
| 11 | NT | NT | NT | >100 |
| 12 | NT | NT | NT | 96.4 ± 2.35 |
| 13 | NT | NT | NT | 98.0 ± 2.36 |
| 14 | 19.1 ± 0.8 | NT | NT | 32.88 ± 2.46 |
| 15 | 30.6 ± 2.0 | NT | NT | 46.91 ± 2.74 |
| 16 | NT | NT | NT | >100 |
| 17 | 53.8 ± 1.2 | NT | NT | ND |
| 18 | NT | NT | NT | 21.53 ± 2.37 |
| 19 | NT | NT | 31.59 ± 0.44 | >100 |
| 20 | 37.0 ± 1.4 | NT | NT | 31.64 ± 2.20 |
| 21 | NT | 86.36 ± 0.93 | 33.09 ± 2.34 | 52.4 ± 2.25 |
| 22 | NT | NT | NT | >100 |
| AUDAb | 10.9 ± 1.2 nM | — | — | — |
| Tacrineb | — | 207.84 ± 4.29 nM | 7.46 ± 0.86 nM | — |
| Apigeninb | — | — | — | 1.64 ± 0.19 |
 | ||
| Fig. 4 Lineweaver–Burk plot for the sEH inhibition of compound 14 (A). Dixon plot of sEH inhibition by 14 (B). | ||
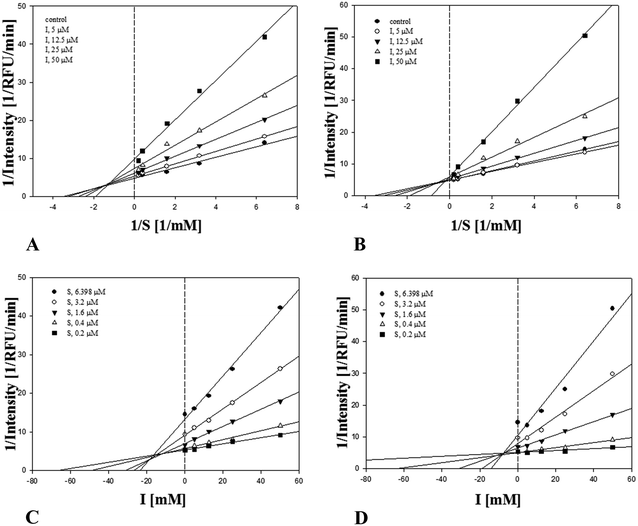 | ||
| Fig. 5 Lineweaver–Burk plot for BChE inhibition of compounds 19 (A) and 21 (B); Dixon plot of BChE inhibition by 19 (C) and 21 (D). | ||
Furthermore, compounds 1–22 (except 9) were assessed for their effects on TNFα-induced NF-κB transcriptional activity in human hepatocarcinoma (HepG2) cells using an NF-κB-luciferase assay. As shown in Table 2, among the tested compounds, only compound 18 significantly inhibited TNFα-induced NF-κB transcriptional activity with IC50 values of 21.53 ± 2.37 μM, while others were weak little or inactive. Apigenin (IC50 1.64 μM) was used as a positive control. To date, there is no literature on the anti-inflammatory effects of compound 18. These results led us to conclude that ursolic acid lactone (14) and 9α-hydroxysesamin (18) from the seeds of Catalpa bungei exhibit significant anti-inflammatory effects by inhibition of sEH enzyme and TNFα-induced NF-κB activation, and that D-pinoresinol (19) and ladanein (21) inhibit BChE. These results also provide scientific support for the use of Catalpa bungei in the prevention of cancer, inflammatory and neurodegenerative diseases.
In conclusion, three cage-like chlorinated iridoid glucosides (1–3) including two new bungoside A (1) and bungoside B (2), as well as 19 compounds, were isolated from the seeds of Catalpa bungei. Among the isolates, ursolic acid lactone (14) showed a potential inhibitory activity against soluble epoxide hydrolase (sEH), and 9α-hydroxysesamin (18) significantly inhibited TNFα-induced NF-κB transcriptional activity. Furthermore, D-pinoresinol (19) and ladanein (21) displayed marked inhibitory effects on BChE. Our observations provide scientific support for the use of Catalpa bungei in the prevention of cancer, inflammatory and neurodegenerative diseases.
Experimental
General experimental procedures
Optical rotations were measured with a DKSH Automatic A21101 polarimeter. UV spectra were obtained using a Thermo Scientific Evolution 300 UV-VIS Spectrophotometer. 1H and 13C NMR spectra were recorded on 400 MHz and 500 MHz NMR instruments (Bruker Daltonics Inc., Bremen, Germany). Chemical shifts were reported using solvent residual peak as the internal standard. ESI-MS spectra were performed on a Thermo Fisher LTQ Fleet instrument spectrometer (Thermo Fisher Scientific Inc., MA, U.S.). Column chromatography (CC) was performed on silica gel (90–150 μm) (Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (40–70 μm) (Amersham Pharmacia Biotech AB, Uppsala, Sweden), and Lichroprep RP-18 gel (40–63 μm; Merck, Darmstadt, Germany). GF254 plates (Qingdao Marine Chemical Inc., Qingdao, China) were used for thin-layer chromatography (TLC). Preparative TLC (PTLC) was carried out on silica gel 60 GF254 (Qingdao Marine Chemical, Ltd., Qingdao, China); semi-preparative HPLC (Waters Corp., Milford) was performed by a Thermo BDS Hypersil column (250 × 4.6 mm and 250 × 10 mm, 5 μm) (Thermo Fisher Scientific Inc., MA).Acetylcholinesterase (AChE) from electric eel (Type-VI-S, E.C. 3.1.1.7), acetylthiocholine iodide (ATChI), butyrylcholinesterase (BChE) from equine serum (E.C. 3.1.1.8), butyrylthiocholine iodide (BTChI), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), tacrine were obtained from Sigma-Aldrich (St. Louis, MO). Potassium phosphate were purchased from Amresco (K2HPO4) and Daejung (KH2PO4).
Plant materials
The seeds of Catalpa bungei C. A. Mey were collected from Lveyang County, Shaanxi Province of China on November 25, 2012. The plant was taxonomically identified by one of authors (K. J. Z.). A voucher specimen (LXY-01239) has been deposited at the Herbarium of College of Life Sciences, Northwest A&F University, P. R. China.Extraction and isolation
The dried and powdered seeds (20 kg) of C. bungei were extracted three times with 75% EtOH at room temperature. The solvent was evaporated under reduced pressure to obtain a crude EtOH extract (1.042 g), which was suspended in H2O and partitioned successively with petroleum ether (PE), EtOAc, and n-BuOH. The EtOAc fraction (ca. 250 g) was chromatographed on silica gel over CC eluting with EtOAc/MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15
1, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give five fractions 1–5. Fraction 4 (16 g) was subjected to silica gel CC using CHCl3/MeOH (10
1) to give five fractions 1–5. Fraction 4 (16 g) was subjected to silica gel CC using CHCl3/MeOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give four fractions 4.1–4.4. Fraction 4.1 was fractionated on Sephadex LH-20 with MeOH and then further purified by a silica gel column using PE/acetone (7
1) to give four fractions 4.1–4.4. Fraction 4.1 was fractionated on Sephadex LH-20 with MeOH and then further purified by a silica gel column using PE/acetone (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 10 (17 mg). Fraction 4.2 was loaded to silica gel CC using CHCl3/MeOH (5
1) to afford 10 (17 mg). Fraction 4.2 was loaded to silica gel CC using CHCl3/MeOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–2
1–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to give four fractions (4.2.1–4.2.4). Fraction 4.2.2 was purified by RP-18 CC using MeOH/H2O (0
1) as eluent to give four fractions (4.2.1–4.2.4). Fraction 4.2.2 was purified by RP-18 CC using MeOH/H2O (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–5
10–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as a solvent to give 4 (88 mg) and 15 (27 mg). Fraction 4.2.3 was rechromatographed over RP-18 column (30% methanol) and further purified by RP-HPLC using MeOH/H2O (25
5) as a solvent to give 4 (88 mg) and 15 (27 mg). Fraction 4.2.3 was rechromatographed over RP-18 column (30% methanol) and further purified by RP-HPLC using MeOH/H2O (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, flow rate: 3 mL min−1) to afford 1 (18.5 mg, tR = 37.5 min) and 2 (17.1 mg, tR = 42.5 min).
75, flow rate: 3 mL min−1) to afford 1 (18.5 mg, tR = 37.5 min) and 2 (17.1 mg, tR = 42.5 min).
Fraction 4.3 was separated by CC over RP-18 with 10–50% MeOH. Subfraction eluted with 50% MeOH was purified by silica gel CC eluting with CHCl3/MeOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by semi-preparative RP-HPLC using MeOH/H2O (40
1), followed by semi-preparative RP-HPLC using MeOH/H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60; flow rate = 2 mL min−1) to obtain 3 (30 mg, tR = 18.5 min).
60; flow rate = 2 mL min−1) to obtain 3 (30 mg, tR = 18.5 min).
Fraction 4.4 was separated by a Sephadex LH-20 column and then RP-18 CC. Its water fraction was purified on CC over silica gel with CHCl3/MeOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to give 5 (9 mg).
1) as eluent to give 5 (9 mg).
Fraction 1 was applied to a silica gel column by elution with EtOAc/MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5
1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to generate three fractions (1.1–1.3). Fraction 1.1 was loaded to medium pressure liquid chromatography over RP-C18 by gradient elution with MeOH/H2O (40–100%) to furnish 6 fractions (1.1.1–1.1.6). Fraction 1.1.1 eluted by 40% MeOH was purified by Sephadex LH-20 to give 22 (90 mg). Fraction 1.1.2 (eluted by 50% MeOH) was purified by Sephadex LH-20 and then by CC over silica gel and eluted by CHCl3/MeOH (10
1) to generate three fractions (1.1–1.3). Fraction 1.1 was loaded to medium pressure liquid chromatography over RP-C18 by gradient elution with MeOH/H2O (40–100%) to furnish 6 fractions (1.1.1–1.1.6). Fraction 1.1.1 eluted by 40% MeOH was purified by Sephadex LH-20 to give 22 (90 mg). Fraction 1.1.2 (eluted by 50% MeOH) was purified by Sephadex LH-20 and then by CC over silica gel and eluted by CHCl3/MeOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 17 (14.5 mg). Fraction 1.1.3 was separated successively by Sephadex LH-20, RP-18 CC (45% MeOH) and then silica gel CC (CHCl3/MeOH 5
1) to obtain 17 (14.5 mg). Fraction 1.1.3 was separated successively by Sephadex LH-20, RP-18 CC (45% MeOH) and then silica gel CC (CHCl3/MeOH 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 20 (6.5 mg). Fraction 1.1.4 was submitted to Sephadex LH-20 to furnish three fractions (1.1.4.1–1.1.4.3). Fraction 1.1.4.1 was purified by CC on silica gel eluting with CHCl3/MeOH (7
1) to give 20 (6.5 mg). Fraction 1.1.4 was submitted to Sephadex LH-20 to furnish three fractions (1.1.4.1–1.1.4.3). Fraction 1.1.4.1 was purified by CC on silica gel eluting with CHCl3/MeOH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 18 (20.0 mg). Fraction 1.1.4.2 was purified by CC on silica gel and further Sephadex LH-20 to provide 19 (9.0 mg). Fraction 1.1.4.3 was recrystallized to give 21 (20 mg) in MeOH. Fraction 1.1.5 (80% MeOH) was separated successively by CC over Sephadex LH-20, RP-18, and silica gel (CHCl3/MeOH 50
1) to give 18 (20.0 mg). Fraction 1.1.4.2 was purified by CC on silica gel and further Sephadex LH-20 to provide 19 (9.0 mg). Fraction 1.1.4.3 was recrystallized to give 21 (20 mg) in MeOH. Fraction 1.1.5 (80% MeOH) was separated successively by CC over Sephadex LH-20, RP-18, and silica gel (CHCl3/MeOH 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce 12 (11.6 mg) and 13 (6.7 mg). Fraction 1.1.6 was purified successively by Sephadex LH-20, RP-18 (85% MeOH), and CC on silica gel (CHCl3/MeOH 20
1) to produce 12 (11.6 mg) and 13 (6.7 mg). Fraction 1.1.6 was purified successively by Sephadex LH-20, RP-18 (85% MeOH), and CC on silica gel (CHCl3/MeOH 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afforded 14 (7.0 mg).
1) to afforded 14 (7.0 mg).
Fraction 1.2 was fractionated successively by CC over silica gel, Sephadex LH-20, and RP-18 (70% MeOH) and then by silica gel CC (CHCl3/MeOH 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 11 (9.3 mg). Fraction 3 was separated by reduced pressure CC over RP-18 eluting by using MeOH/H2O in a gradient elution (from 1
1) to give 11 (9.3 mg). Fraction 3 was separated by reduced pressure CC over RP-18 eluting by using MeOH/H2O in a gradient elution (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 5
10 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to yield six fractions (3.1–3.6). Fraction 3.1 was separated by Sephadex LH-20 and silica gel CC using CHCl3/MeOH (20
5) to yield six fractions (3.1–3.6). Fraction 3.1 was separated by Sephadex LH-20 and silica gel CC using CHCl3/MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6 (9 mg). Fraction 3.2 was separated by Sephadex LH-20 to furnish two fractions (3.2.1–3.2.2). Fraction 3.2.1 was purified by semi-preparative RP-HPLC using MeOH/H2O (40
1) to give 6 (9 mg). Fraction 3.2 was separated by Sephadex LH-20 to furnish two fractions (3.2.1–3.2.2). Fraction 3.2.1 was purified by semi-preparative RP-HPLC using MeOH/H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60; flow rate: 3 mL min−1) to give 9 (5.5 mg, tR = 18.5 min) and 8 (13.5 mg, tR = 19.3 min). Compound 7 (15.1 mg) was isolated from fraction 3.2.2 by using silica gel CC eluted with CHCl3/MeOH (8.5
60; flow rate: 3 mL min−1) to give 9 (5.5 mg, tR = 18.5 min) and 8 (13.5 mg, tR = 19.3 min). Compound 7 (15.1 mg) was isolated from fraction 3.2.2 by using silica gel CC eluted with CHCl3/MeOH (8.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and compound 16 (181 mg) was obtained from fraction 3.3 using Sephadex LH-20 and silica gel CC eluting with CHCl3/MeOH/H2O (4
1), and compound 16 (181 mg) was obtained from fraction 3.3 using Sephadex LH-20 and silica gel CC eluting with CHCl3/MeOH/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02).
0.02).
The purity of every purified chemicals was more than 98% by HPLC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 328 nm (4.28); IR (KBr) νmax 3393, 1697, 1630, 1598, 1516 cm−1; 1H and 13C NMR data, see Table 1. ESI-MS (positive) m/z 597.49 [M + Na]+; HR-ESI-MS m/z 597.1343 ([M + Na]+, calcd C25H31ClO13Na+, 597.1351).
ε) 328 nm (4.28); IR (KBr) νmax 3393, 1697, 1630, 1598, 1516 cm−1; 1H and 13C NMR data, see Table 1. ESI-MS (positive) m/z 597.49 [M + Na]+; HR-ESI-MS m/z 597.1343 ([M + Na]+, calcd C25H31ClO13Na+, 597.1351).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 316 nm (4.23); IR (KBr) νmax 3383, 1690, 1631, 1604, 1514 cm−1; 1H and 13C NMR data, see Table 1. ESI-MS (positive) m/z 567.23 [M + Na]+; HR-ESI-MS m/z 567.1243 ([M + Na]+, calcd C24H29ClO12Na+, 567.1245).
ε) 316 nm (4.23); IR (KBr) νmax 3383, 1690, 1631, 1604, 1514 cm−1; 1H and 13C NMR data, see Table 1. ESI-MS (positive) m/z 567.23 [M + Na]+; HR-ESI-MS m/z 567.1243 ([M + Na]+, calcd C24H29ClO12Na+, 567.1245).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 261 nm (4.37); IR (KBr) νmax 3400, 1693, 1607, 1514 cm−1; 1H and 13C NMR data, see Table 1. ESI-MS (positive) m/z 541.38 [M + Na]+; HR-ESI-MS m/z 541.1081 ([M + Na]+, calcd C22H27ClO12Na+, 541.1089).
ε) 261 nm (4.37); IR (KBr) νmax 3400, 1693, 1607, 1514 cm−1; 1H and 13C NMR data, see Table 1. ESI-MS (positive) m/z 541.38 [M + Na]+; HR-ESI-MS m/z 541.1081 ([M + Na]+, calcd C22H27ClO12Na+, 541.1089).Acetylation of 3
Compound 3 (3 mg) was treated with acetic anhydride (0.2 mL) in dry pyridine (0.2 mL) at room temperature. The mixture was stirred for 24 h, on workup to give a pure derivative 3a (ca. 3 mg).![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3), 170.4 (C–
OCH3), 170.4 (C–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3), 167.0 (C–
OCH3), 167.0 (C–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3), 169.5 (C–
OCH3), 169.5 (C–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3), 169.21 (C–
OCH3), 169.21 (C–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3), 168.9 (C–
OCH3), 168.9 (C–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) OCH3), 165.7 (C-11), 154.8 (C-15), 131.6 (C-13,17), 127.0 (C-12), 121.9 (C-14, 16), 95.3 (C-1′), 94.5 (C-1), 91.9 (C-3), 86.2 (C-6), 85.9 (C-8), 72.9 (C-3′), 72.2 (C-5′), 71.04 (C-2′), 68.3 (C-4′), 63.0 (C-7), 61.7 (C-6′), 60.0 (C-10), 42.0 (C-9), 33.5 (C-5), 32.9 (C-4), 22.3 (C–CO
OCH3), 165.7 (C-11), 154.8 (C-15), 131.6 (C-13,17), 127.0 (C-12), 121.9 (C-14, 16), 95.3 (C-1′), 94.5 (C-1), 91.9 (C-3), 86.2 (C-6), 85.9 (C-8), 72.9 (C-3′), 72.2 (C-5′), 71.04 (C-2′), 68.3 (C-4′), 63.0 (C-7), 61.7 (C-6′), 60.0 (C-10), 42.0 (C-9), 33.5 (C-5), 32.9 (C-4), 22.3 (C–CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), 21.3 (C–CO
H3), 21.3 (C–CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), 20.9 (C–CO
H3), 20.9 (C–CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), 20.8 (C–CO
H3), 20.8 (C–CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3), 20.7 (C-2 × CO
H3), 20.7 (C-2 × CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H3); 1H NMR (500 MHz, CDCl3) δ 8.08 (2H, d, J = 8.7 Hz, H-13, 17), 7.20 (2H, d, J = 8.7 Hz, H-14,16), 5.58 (1H, d, J = 2.0 Hz, H-1), 5.49 (1H, d, J = 8.0 Hz, H-7), 5.39 (1H, d, J = 2.7 Hz, H-3), 5.22 (1H, ddd, J = 9.4, 7.3, 2.2 Hz, 3′), 5.17 (1H, dd, J = 8.0, 2.8 Hz, H-6), 5.11 (1H, t, J = 9.7 Hz, H-4′), 4.96 (2H, m, H-1′,2′), 4.31 (2H, m, H-6′b,10α), 4.15 (1H, dd, J = 12.4, 2.3 Hz, H-6′a), 3.90 (1H, d, J = 12.5 Hz, H-10β), 3.73 (1H, ddd, J = 10.0, 4.1, 2.4 Hz, H-5′), 3.60 (1H, br d, J = 10.3 Hz, H-9), 2.40 (1H, m, H-5), 2.33 (3H, s, H–CO
H3); 1H NMR (500 MHz, CDCl3) δ 8.08 (2H, d, J = 8.7 Hz, H-13, 17), 7.20 (2H, d, J = 8.7 Hz, H-14,16), 5.58 (1H, d, J = 2.0 Hz, H-1), 5.49 (1H, d, J = 8.0 Hz, H-7), 5.39 (1H, d, J = 2.7 Hz, H-3), 5.22 (1H, ddd, J = 9.4, 7.3, 2.2 Hz, 3′), 5.17 (1H, dd, J = 8.0, 2.8 Hz, H-6), 5.11 (1H, t, J = 9.7 Hz, H-4′), 4.96 (2H, m, H-1′,2′), 4.31 (2H, m, H-6′b,10α), 4.15 (1H, dd, J = 12.4, 2.3 Hz, H-6′a), 3.90 (1H, d, J = 12.5 Hz, H-10β), 3.73 (1H, ddd, J = 10.0, 4.1, 2.4 Hz, H-5′), 3.60 (1H, br d, J = 10.3 Hz, H-9), 2.40 (1H, m, H-5), 2.33 (3H, s, H–CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.29 (1H, m, H-4β), 2.11, 2.10, 2.02, 2.01, 1.99 (each 3H, s, H–CO
3), 2.29 (1H, m, H-4β), 2.11, 2.10, 2.02, 2.01, 1.99 (each 3H, s, H–CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.06 (1H, m, H-4α).
3), 2.06 (1H, m, H-4α).Acid hydrolysis of 1–3 and determination of sugar
Compounds 1–3 (2.0 mg, each) was hydrolysed with 2 M HCl–dioxane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) at 100 °C for 2 h. The reaction mixture was partitioned between chloroform and H2O three times. The aqueous layer was neutralized with 2 M Ag2CO3 and evaporated in vacuo. The residue was dissolved in pyridine (0.5 mL), to which L-cysteine methyl ester hydrochloride in pyridine (0.1 M, 0.5 mL) was added. After reacting at 60 °C for 1 h, trimethylsilylimidazole (0.05 mL) was added to the reaction mixture and kept at 4 °C for another 8 h. The mixture was analysed by GC-MS. By comparison of the retention time of the authentic sample, the monosaccharide of compounds 1–3 was determined to be D-glucose (tR = 11.85 min) (tR = 12.15 min of L-glucose).
1, 1 mL) at 100 °C for 2 h. The reaction mixture was partitioned between chloroform and H2O three times. The aqueous layer was neutralized with 2 M Ag2CO3 and evaporated in vacuo. The residue was dissolved in pyridine (0.5 mL), to which L-cysteine methyl ester hydrochloride in pyridine (0.1 M, 0.5 mL) was added. After reacting at 60 °C for 1 h, trimethylsilylimidazole (0.05 mL) was added to the reaction mixture and kept at 4 °C for another 8 h. The mixture was analysed by GC-MS. By comparison of the retention time of the authentic sample, the monosaccharide of compounds 1–3 was determined to be D-glucose (tR = 11.85 min) (tR = 12.15 min of L-glucose).
Biological assays
| sEH inhibitory activity (%) = 100 − [(S60 − S0)/(C60 − C0)] × 100 |
Computational section
MMFF and DFT calculations were run with Spartan'14 (Wavefunction, Inc., Irvine CA, 2014), with standard parameters and convergence criteria. TDDFT calculations were run with Gaussian'09,51 with default grids and convergence criteria. Conformational searches were run with the Monte Carlo algorithm implemented in Spartan'14 using Merck molecular force field (MMFF). All structures thus obtained were optimized with DFT method using B3LYP functional and 6-31G(d) basis set in vacuo. TDDFT calculations were run using B3LYP functional and TZVP basis set, including 24 excited states. On some selected structures, other functionals (CAM-B3LYP, PBE0, M06, M06-2X) and basis sets (augmented TZVP, ADZP) were also tested, leading to consistent results. UV and CD spectra were generated using the program SpecDis.52 by applying a Gaussian band shape with 0.2 eV exponential half-width, from dipole-length rotational strengths.Acknowledgements
This work was financially supported by the National Forestry Public Welfare Industry Research Project (201204603) and the Program of Science & Technology of Shaanxi Province (2013SF2-15) as well as by the Priority Research Center Program (2009-0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea. T. I. gratefully acknowledges FP7-REGPOT-2012-2013-1, grant agreement number 316289–InnoMol.Notes and references
- J. D. Imig and B. D. Hammock, Nat. Rev. Drug Discovery, 2009, 8, 794–805 CrossRef CAS PubMed.
- W. B. Campbell, D. Gebremedhin, P. F. Pratt and D. R. Harder, Circ. Res., 1996, 78, 415–423 CrossRef CAS PubMed.
- P. T. Francis, A. M. Palmer, M. Snape and G. K. Wilcock, J. Neurol., Neurosurg. Psychiatry, 1999, 66, 137–147 CrossRef CAS.
- E. Giacobini, Pharmacol. Res., 2004, 50, 433–440 CrossRef CAS PubMed.
- L. Tornatore, A. K. Thotakura, J. Bennett, M. Moretti and G. Franzoso, Trends Cell Biol., 2012, 22, 557–566 CrossRef CAS PubMed.
- Z. H. Wu and S. Miyamoto, J. Mol. Med., 2007, 85, 1187–1202 CrossRef CAS PubMed.
- N. S. Rial, K. Choi, T. Nguyen, B. Snyder and M. J. Slepian, Med. Hypotheses, 2012, 78, 29–32 CrossRef CAS PubMed.
- (a) E. Kanai, K. Machida and M. Kikuchi, Chem. Pharm. Bull., 1996, 44, 1607–1609 CrossRef CAS; (b) A. Fujiwara, T. Mori, A. Iida, S. Ueda, Y. Hano, T. Nomura, H. Tokuda and H. Nishino, J. Nat. Prod., 1998, 61, 629–632 CrossRef CAS PubMed; (c) K. Machida, E. Hishinuma and M. Kikuchi, Chem. Pharm. Bull., 2004, 52, 618–621 CrossRef CAS PubMed; (d) J. Y. Cho, H. Y. Kim and G. J. Choi, Pest Manage. Sci., 2006, 62, 414–418 CrossRef CAS PubMed.
- J. H. Kuk, S. J. Ma, J. H. Moon, K. Y. Kim, S. H. Choi and K. H. Park, J. Microbiol. Biotechnol., 2002, 12, 858–863 Search PubMed.
- F. Dal Piaz, A. Vassallo, A. Temraz, R. Cotugno, M. A. Belisario, G. Bifulco, M. G. Chini, C. Pisano, N. De Tommasi and A. Braca, J. Med. Chem., 2013, 56, 1583–1595 CrossRef PubMed.
- K. Machida, M. Ogawa and M. Kikuchi, Chem. Pharm. Bull., 1998, 46, 1056–1057 CrossRef CAS.
- W. T. Lee, Coloured standard illustration of Korean plants, Academy Publishing Co., 1996, p. 326 Search PubMed.
- J. J. Tang, F. Y. Zhang, D. M. Wang, J. M. Tian, S. Dong and J. M. Gao, Int. J. Mol. Sci., 2013, 14, 19484–19493 CrossRef CAS PubMed.
- H. W. Liu, X. Z. Yu, D. Padula, G. Pescitelli, Z. W. Lin, F. Wang, K. Ding, M. Lei and J. M. Gao, Eur. J. Med. Chem., 2013, 59, 265–273 CrossRef CAS PubMed.
- (a) D. M. Wang, C. C. Zhang, Q. Zhang, N. Shafiq, G. Pescitelli, D. W. Li and J. M. Gao, J. Agric. Food Chem., 2014, 62, 6669–6676 CrossRef CAS PubMed; (b) J. M. Gao, W. J. Wu and J. W. Zhang, Nat. Prod. Rep., 2007, 24, 1153–1189 RSC.
- (a) M. M. Bai, W. Shi, J. M. Tian, M. Lei, Y. H. Kim, Y. N. Sun, J. H. Kim and J. M. Gao, J. Agric. Food Chem., 2015, 63, 2198–2205 CrossRef CAS PubMed; (b) H. Li, J. M. Tian, H. Y. Tang, S. Y. Pan, A. L. Zhang and J. M. Gao, RSC Adv., 2015, 5, 29185–29192 RSC.
- C. A. Boros and F. R. Stermitz, J. Nat. Prod., 1991, 54, 1173–1246 CrossRef CAS.
- K. Machida, C. Ikeda, R. Kakuda, Y. Yaoita and M. Kikuchi, Nat. Med., 2001, 55, 61–63 CAS.
- T. Kaneko, K. Ohtani, R. Kasai, K. Yamasaki and M. D. Nguyen, Phytochemistry, 1998, 47, 259–263 CrossRef CAS.
- M. Wang, J. Li, M. Rangarajan, Y. Shao, E. J. LaVoie, T. C. Huang, C. T. Ho and J. Agric, Food Chem., 1998, 46, 4869–4873 CrossRef CAS.
- H. Shimomura, Y. Sashida and T. Adachi, Phytochemistry, 1988, 27, 641–644 CrossRef CAS.
- S. X. Yang, J. M. Gao, J. C. Qin, L. Zhou, M. H. Chiu and L. Wang, Helv. Chim. Acta, 2007, 90, 1477–1481 CrossRef CAS.
- F. P. Soares, C. A. V. Ronconi, E. V. L. da Cunha, J. M. Barbosa-Filho, M. S. da Silva and R. Braz-Filho, Magn. Reson. Chem., 1998, 36, 608–614 CrossRef CAS.
- B. K. Ponou, R. B. Teponno, M. Ricciutelli, T. B. Nguelefack, L. Quassinti, M. Bramucci, G. Lupidi, L. Barboni and L. A. Tapondjou, Chem. Biodiversity, 2011, 8, 1301–1309 CAS.
- T. H. Lee, S. S. Lee, Y. C. Kuo and C. H. Chou, J. Nat. Prod., 2001, 64, 865–869 CrossRef CAS.
- N. M. Al Musayeib, R. A. Mothana, A. A. Gamal, S. M. Al-Massarani and L. Maes, Molecules, 2013, 18, 9207–9218 CrossRef CAS PubMed.
- T. Ersoz, D. Tasdemir, I. Calis and C. M. Ireland, Turk. J. Chem., 2002, 26, 465–471 CAS.
- B. Gering and M. Wichtl, J. Nat. Prod., 1987, 50, 1048–1054 CrossRef CAS.
- S. H. Wu, Y. M. Shen, Y. W. Chen, Z. Y. Li, L. Y. Yang and S. L. Li, Chem. Nat. Compd., 2009, 45, 536–538 CrossRef CAS.
- A. L. Sousa, Q. S. Sales, R. Braz-Filho and R. R. de Oliveira, J. Liq. Chromatogr. Relat. Technol., 2012, 35, 2294–2303 CAS.
- L. H. Xie, T. Akao, K. Hamasaki, T. Deyama and M. Hattori, Chem. Pharm. Bull., 2003, 51, 508–515 CrossRef CAS PubMed.
- H. Itokawa, K. Matsumoto, H. Morita and K. Takeya, Phytochemistry, 1992, 31, 1061–1062 CrossRef CAS.
- M. H. Farjam, A. Rustaiyan, E. Ezzatzadeh and A. R. Jassbi, Iran. J. Pharm. Res., 2013, 12, 395–400 CAS.
- A. Andolfi, A. Boari, M. Evidente, A. Cimmino, M. Vurro, G. Ash and A. Evidente, J. Nat. Prod., 2015, 78, 623–629 CrossRef CAS PubMed.
- C. A. Boros and F. R. Stermitz, J. Nat. Prod., 1990, 53, 1055–1147 CrossRef CAS.
- X. P. Yang, E. W. Li, Q. Zhang, C. S. Yuan and Z. J. Jia, Chem. Biodiversity, 2006, 3, 762–770 CAS.
- R. Wang, D. Xiao, Y. H. Bian, X. Y. Zhang, B. J. Li, L. S. Ding and S. L. Peng, J. Nat. Prod., 2008, 71, 1254–1257 CrossRef CAS PubMed.
- R. K. Chaudhuri, F. Ü. Afifi-Yazar and O. Sticher, Helv. Chim. Acta, 1979, 62, 1603–1604 CrossRef CAS.
- R. K. Chaudhuri and O. Sticher, Tetrahedron Lett., 1979, 34, 3149–3152 CrossRef.
- S. K. Ling, T. Tanaka and I. Kouno, J. Nat. Prod., 2001, 64, 796–798 CrossRef CAS.
- Q. Jia, M. F. Hong and D. Minter, J. Nat. Prod., 1999, 62, 901–903 CrossRef CAS PubMed.
- T. Iwagawa, T. Hamada, S. Kurogi, T. Hase, T. Okubo and M. Kim, Phytochemistry, 1991, 30, 4057–4060 CrossRef CAS.
- M. Yoshikawa, Y. Fukuda, T. Taniyama and I. Kitagawa, Chem. Pharm. Bull., 1986, 34, 1403–1406 CrossRef CAS.
- K. Weinges, H. Schick, K. Neuberger, H. J. Ziegler, J. Lichtenthäler and H. Irngartinger, Liebigs Ann. Chem., 1989, 11, 1113–1122 CrossRef.
- I. Kitagawa, Y. Fukuda, T. Taniyama and M. Yoshikawa, Chem. Pharm. Bull., 1995, 43, 1096–1100 CrossRef CAS.
- J. Autschbach, L. Nitsch-Velasquez and M. Rudolph, Top. Curr. Chem., 2011, 298, 1–98 CrossRef CAS PubMed.
- S. Iwahana, H. Iida, E. Yashima, G. Pescitelli, L. Di Bari, A. G. Petrovic and N. Berova, Chem.–Eur. J., 2014, 20, 4386–4395 CrossRef CAS PubMed.
- H. Wang, F. H. Wu, F. Xiong, J. J. Wu, L. Y. Zhang, W. C. Ye, P. Li and S. X. Zhao, Chem. Pharm. Bull., 2006, 54, 1144–1149 CrossRef CAS PubMed.
- I. Kitagawa, Y. Fukuda, T. Taniyama and M. Yoshikawa, Chem. Pharm. Bull., 1991, 39, 1171–1176 CrossRef CAS.
- G. L. Ellman, K. D. Courtney Jr. and V. Andres, et al., Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
- T. Bruhn, A. Schaumlöffel, Y. Hemberger and G. Bringmann, Chirality, 2013, 25, 243–249 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: HR-ESI-MS, NMR, and IR spectra of compounds 1–3, bioactive data of compounds. See DOI: 10.1039/c6ra04207d |
| This journal is © The Royal Society of Chemistry 2016 |