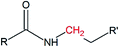
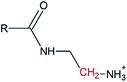
Solid-state polymerization of EDTA and ethylenediamine as one-step approach to monodisperse hyperbranched polyamides†
V. A. Tomazab,
A. F. Rubira*ab and
R. Silva *a
*a
aDepartment of Chemistry, State University of Maringá (UEM), Av. Colombo 5790, 87020 – Maringá, Paraná, Brazil. E-mail: afrubira@uem.br; rsilva2@uem.br; Tel: +55 4430113686 Tel: +55 4430113664
bGrupo de Materiais Poliméricos e Compósitos, State University of Maringá, Av. Colombo 5790, 87020 – Maringá, Paraná, Brazil
First published on 19th April 2016
Abstract
Hyperbranched polyamides (HBPAs), a special class of polymers with extended use in colloidal systems, is synthesized by an innovative method taking advantage of the ordered aggregation of positive and negative molecular ions in solid-state. This novel and facile procedure introduces a one-step approach to convert ethylenediaminetetraacetic acid (EDTA) and ethylenediamine (EDA) in macromolecules with ordered framework. EDTA and EDA are converted in negative and positive molecular ions through acid–base reaction. Then, Coulomb interaction among the charged molecules are used to drive the aggregation of the ions during the precipitation induced by casting process. The solid material composed by the molecular ions are subjected to thermal treatment to promote amide formation reaction in solid-state. The synthesis condition is evaluated to understand the macromolecule growth. Solid-state reaction products are chemically characterized by FTIR and 1H and 13C NMR. Molecular weight is determined by gel permeation chromatography (GPC) and the particles diameter in solution and charges are measured by dynamic light scattering (DLS) and zeta potential, respectively, at different pH values. The results attested the synthesis of hyperbranched polyamides with features similar to polyamide dendrimers. Surprisingly, the method enables the synthesis of macromolecules with very low dispersity index (DPI), that in some cases can be as low as 1.1.
Introduction
Hyperbranched macromolecules, such as star-polymers and dendrimers, have been playing important roles in the development of applied systems in colloidal chemistry, since they possess a vast list of potential applications due their unique characteristics in solution. For instance, as excellent host material to stabilize metal nanoparticles or as biomaterials to promote special interactions with biomembranes.1–4 A remarkable feature of hyperbranched macromolecules is the high content of terminal groups.5–7 These terminals groups can be easily converted to address the design of desired systems, affecting their solubility in different solvents, biocompatibility and reactivity.8–10 In addition, stable conformations assumed by hyperbranched macromolecules in solutions provide outstanding capping properties for nanoparticles or drugs.11–14 As a consequence of their remarkable properties in solution, hyperbranched macromolecules are widely explored in nanotechnology, biomedicine, coatings process and resins formulations.15–17A large diversity of hyperbranched macromolecules were previously reported.18–22 Despite the large diversity of structures, two main classes of hyperbranched macromolecules emerge as more prominent: dendrimers and hyperbranched polymers. Dendrimers are perfectly branched and monodisperse macromolecules. Dendrimers are prepared by complex synthesis approaches of successive cyclic steps that end up in expensive materials and therefore, materials that are not attractive for large-scale industrial process.23,24 On the other hand, hyperbranched polymers are usually prepared through one-pot synthesis. The issue of one-pot synthesis is the limited control on molecular weight and branching accuracy. Therefore, hyperbranched polymers are heterogeneous materials in terms of molecular weight and branching.25,26 Despite the more heterogeneous structure, hyperbranched polymers have the facile synthesis as their great advantage, which ease their fabrication in large amount at reduced cost. Hence, they can be thought to be used in general applications.
The comparison between dendrimers and hyperbranched polymers rises a challenge in polymer science, regarding the development of systems to combine the advantages from these two polymers classes. A convergence point between dendrimers and hyperbranched polymer would result in outstanding macromolecules by a technology point of view. The convergence point should correlate very simple synthesis and purification procedures to result in dendritic polymers with well-defined molecular size and shape structure.
Organic solid-state reactions are normally uncontrolled processes with very few examples in the literature.27–29 Inherent solid-state features, what could be thought to drive reactions to be hardly controlled, such as low reactants diffusion and degree of freedom, can be explored in a way to favor the formation of organized frameworks. Molecules with specific and strong intermolecular interaction, aggregate in the solid-state forming crystals, where the orientation of the molecules are assumed to benefit the attractive forces. Quasi-static location of molecules in a crystal in relation to their neighborhood could indeed be explored to drive reaction at specific molecular positions. It could be done by placing reactive groups of different molecules in close proximity to favor a determined reaction.
The methodology based in the use of aggregated reactants organized in the solid-state to favor the formation of macromolecules with organized framework is for the first time demonstrated in the present work. Herein, the preparation of hyperbranched polyamidoamine by a single reaction step procedure is presented and discussed. The materials prepared have chemical structures akin to the well-known polyamidoamine dendrimers, known as PAMAM,30,31 which is a widely studied dendrimers in fields of drug delivery and nanoparticles preparation.32,33
Experimental
Precursor salt
Ethylenediaminetetraacetic acid (EDTA) and ethylenediamine (EDA) were purchased from Sigma-Aldrich and used as received. EDTA and EDA solution were prepared using deionized water.Ethylenediaminetetraacetic acid 1 mol L−1 (EDTA) solution and ethylenediamine 1 mol L−1 (EDA) solution were mixed in proper amounts to prepare solutions with EDTA to EDA ratio, of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 or 1
1.5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. For instance, 100 mL of 1.0 mol L−1 EDTA solution was poured into 150 mL of 1.0 mol L−1 solution of EDA. The molecular ratio between EDTA and EDA in the final solution is 1 molecule of EDTA to 1.5 molecules of EDA. Similarly, a solution with EDTA to EDA ratio of 1
2. For instance, 100 mL of 1.0 mol L−1 EDTA solution was poured into 150 mL of 1.0 mol L−1 solution of EDA. The molecular ratio between EDTA and EDA in the final solution is 1 molecule of EDTA to 1.5 molecules of EDA. Similarly, a solution with EDTA to EDA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was also prepared by adding 100 mL of 1.0 mol L−1 EDTA solution into 200 mL of 1.0 mol L−1 of EDA. The formed solutions were dried in a heating plate at constant temperature of 65 °C. In this step, the solvent in a beaker was evaporated without stirring. The solids obtained were transferred to Petri dishes and stored under vacuum in a desiccator using silica beads as desiccant prior their use in the next step.
2 was also prepared by adding 100 mL of 1.0 mol L−1 EDTA solution into 200 mL of 1.0 mol L−1 of EDA. The formed solutions were dried in a heating plate at constant temperature of 65 °C. In this step, the solvent in a beaker was evaporated without stirring. The solids obtained were transferred to Petri dishes and stored under vacuum in a desiccator using silica beads as desiccant prior their use in the next step.
Solid-state polymerization
Solid-state polymerization were carried out by the thermal treatment of the EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA salts. Different thermal treatment conditions were tested. Three different temperature levels (120, 140 and 160 °C) and five thermal treatment time (2, 4, 8, 12 and 24 hours) were used.
EDA salts. Different thermal treatment conditions were tested. Three different temperature levels (120, 140 and 160 °C) and five thermal treatment time (2, 4, 8, 12 and 24 hours) were used.
The solid materials obtained after the thermal treatment were dissolved in deionized water to produced solution having 1 g of polymer in 10 mL of solution. The obtained solutions were purified using a size-exclusion chromatography (SEC) with Sephadex LH-20 as stationary phase. Sephadex are dextran beads with particles size in the range of 25 to 100 μm. Sephadex column were prepared to have 15 cm. Prior the application in the SEC column the solutions were filtered using a sintered glass filter with pore sizes in the range 40 to 100 μm. After the filtration the polymer solution was applied to the column and the column was eluted using distilled water. The first fraction containing around 15% of the applied mass is separated for characterization.
Characterization
Chemical characterization was carried out using FTIR and 1H and 13C NMR. FTIR spectra were recorded in a Bomen MB-100 and analyzed using PeakFit software. 1H and 13C NMR spectra were acquired in a Varian Mercury Plus BB spectrometer, working at 300 and 75.45 MHz, respectively.Gel permeation chromatography (GPC) were carried out to determine the products molar weight (Mn and Mw) and dispersity index. GPC were determined using a gel permeation chromatography GPCmax VE2001 Viscotek model equipped with triple detection system consisting of refractive index (RI), viscosity and light scattering detectors. In the GPC experiment, water was used as the mobile phase under flow rate of 1.0 mL min−1. The integration of the three detectors allowed to obtain the absolute molar weight of the sample.
Dynamic light scattering (DLS) and zeta-potential measurements were carried out at 25 °C using a Brookhaven Instruments Corporation instrument equipped with a He/Ne ion laser (λ = 633 nm). DLS measurements utilized a 90° detection angle: hydrodynamic diameter (Dh) was calculated by the Stokes–Einstein equation, and size distribution was obtained by CONTIN analysis (90Plus/BI-MAS software). Zeta-potential was calculated by the Smoluchowski equation (Zeta Plus Analyzer software). In the DLS and potential measurement, the pH of the polymeric solutions was adjusted with HCl (0.1 mol L−1) or NaOH (0.1 mol L−1).
Results and discussion
Polyamidoamines were obtained by the polymerization of ethylenediaminetetraacetic acid (EDTA) and ethylenediamine (EDA) in solid-state, wherein amide formation reaction causes the condensation of EDTA and EDA. EDTA and EDA are two very widely used substances and both are very well-known as excellent chelating agents. Nevertheless, direct polymerization between EDTA and EDA is not verified in aqueous solution due to acid–base equilibrium. In aqueous solution of EDTA and EDA, acid–base equilibrium favors the neutralization products (K ≫ 1). Therefore, primary amine groups of EDA and carboxylic acid groups of EDTA are mainly present in the form of protonated amine cations and carboxylate anions, as represented in the eqn (1).
 | (1) |
As consequence of above equilibrium, amide formation by the condensation reaction of EDTA and EDA is hindered. The direct use of EDTA and EDA to form polymers cannot be executed in aqueous condition. On the other hand, acid–base equilibrium provides a very interesting opportunity, since acid–base reaction promotes the formation of molecular ions in solution. In the Fig. 1 is presented a schematic representation of the synthetic approach used to obtain hyperbranched polyamide. The acid–base neutralization reaction, that initially difficult amide formation in solution and avoid the polymerization of EDTA and EDA, can be used to the formation of organized macromolecule as represented in the Fig. 1. The molecular ions in solution can be converted into molecular crystals by the simultaneous precipitation of EDTA and EDA. In that case, negatively charged EDTA4− acts as anion specie and positively charged EDA2+ as cation specie. In the experimental procedure, aqueous solutions containing a mixture of EDTA and EDA was heated at 65 °C to allow a slow precipitation process to optimize the formation of a solid crystal dominated by Coulomb interaction among counter ions, and therefore to drive the formation of organized array of EDTA and EDA molecules. The dried materials obtained from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA solutions consist of yellowish and very hygroscope solids. After dried the solid must be kept in a desiccator, otherwise, the solid material obtained quickly absorbs water and becomes a viscous liquid. The strong hygroscope behavior of the solids is attributed to the ionic features of the species associated to a low lattice energy.
EDA solutions consist of yellowish and very hygroscope solids. After dried the solid must be kept in a desiccator, otherwise, the solid material obtained quickly absorbs water and becomes a viscous liquid. The strong hygroscope behavior of the solids is attributed to the ionic features of the species associated to a low lattice energy.
The amide formation reaction can be more effectively carried out in solid-state than in aqueous media, since water is a product of the reaction in the amide formation. In the complete absence of water, the condensation reaction is not a reversible process, therefore higher yields would be easily obtained. The amide reactions occur as demonstrated in the reaction scheme shown in the eqn (2).
 | (2) |
The condensation reactions in solid-state is carried out by thermal activation. The crystals are heated in the temperature range of 120 to 160 °C. Temperatures higher than 160 can cause decomposition of the organic material. The amide formation during the thermal treatment is easily verified in the FTIR spectra of the samples prepared in different EDTA to EDA ratio (Fig. S1–S3†). The spectra of the samples after the thermal treatment evidenced the appearance of a signal at 1680–1630 cm−1, characteristic of amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (νC
O (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups.34 In addition, FTIR spectra present band at 1588 cm−1 characteristic of asymmetric angular deformation of the NH bond of secondary amines (δN–H),35 bands between 2800 and 2947 cm−1 assigned to the stretching vibration of the bond CH of CH2 groups and aliphatic CH3 (ν(s)C–H and ν(as)C–H) and a broadband after 3000 cm−1 which may be associated with the presence of many –OH groups in HBPAs structure.36
O) groups.34 In addition, FTIR spectra present band at 1588 cm−1 characteristic of asymmetric angular deformation of the NH bond of secondary amines (δN–H),35 bands between 2800 and 2947 cm−1 assigned to the stretching vibration of the bond CH of CH2 groups and aliphatic CH3 (ν(s)C–H and ν(as)C–H) and a broadband after 3000 cm−1 which may be associated with the presence of many –OH groups in HBPAs structure.36
A study of variable was carried out to analyze the formation of the amide group and to determine the more suitable condition to the thermal reaction between EDTA and EDA in the solid in function of time and temperature of the thermal treatment. In this step the EDTA/EDA crystals were heated from different times (2, 4, 8, 12 and 24 hours) and temperatures (120, 140 and 160 °C). The reaction conversions were analyzed by FTIR (Fig. S1–S3†). The relative area of the amide νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal were used to evaluating the conversion of aminium and carboxylate groups to amide groups in function of temperature and reaction time. Thus, reaction yield was analyzed between the relative area of the νC
O signal were used to evaluating the conversion of aminium and carboxylate groups to amide groups in function of temperature and reaction time. Thus, reaction yield was analyzed between the relative area of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal of amide and the relative area of the δN–H signal of secondary amine, which was set as a reference, in the spectra of normalized FTIR (Fig. S4 and S5†).
O signal of amide and the relative area of the δN–H signal of secondary amine, which was set as a reference, in the spectra of normalized FTIR (Fig. S4 and S5†).
Comparing the FTIR results, it is possible to verify that the temperature has high influence on the relative area of the amide group at 1660 cm−1. It is observed the amide peak increase with the increase of the thermal treatment. On the other hand, reaction time does not appear to provide practical changes to the relative area of the amide group. To optimize the synthesis and to reduce possible side reactions as consequence of thermal degradation process, the chosen condition to further experiments was the thermal treatment condition with the highest temperature (160 °C) and the shortest time (2 hours).
The materials obtained in the thermal treatment were submitted to a purification step using a cross-linked dextran gel (Sephadex-LH20).37 The Sephadex-LH20 acts as a size exclusion chromatography.38 The spectra of the purified material were different in relation of the thermally treated ones in term of the intensity of the carbonyl band of amide groups. It is verified that the signal of the amide around 1660 cm−1 has a higher intensity in comparison with the amine band around 1600 cm−1 for both EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio, as presented in the Fig. 2.
EDA ratio, as presented in the Fig. 2.
 | ||
Fig. 2 FTIR spectra of HBPAs synthesized from thermal treatment at 160 °C for 2 hours and EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio 1 EDA ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1 1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (i) before and (ii) after purification by SEC. 2 (i) before and (ii) after purification by SEC. | ||
The change in the amide signal intensity clearly indicates that the purified materials have higher content of amide groups in relation to the as-synthesized ones. Since the purified materials have higher molecular weight, because small molecular mass oligomers were removed in the purification process, it is possible to affirm that the growth of the molecular weight during the thermal treatment is directly related to the amide formation reaction. The chemical characterization of the polymers was carried out by NMR. In the Fig. 3 and 4 are presented 1H and 13C NMR spectra for the purified HBPAs prepared with different EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio at 160 °C for 2 hours, respectively. Using NMR spectra of Fig. 3 and 4 and literature data, attributions were made to the major signs.35,39,40
EDA ratio at 160 °C for 2 hours, respectively. Using NMR spectra of Fig. 3 and 4 and literature data, attributions were made to the major signs.35,39,40
 | ||
Fig. 3 1H NMR spectra in D2O for HBPAs obtained at 160 °C for 2 hours from different EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio: (a) 1 EDA ratio: (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and (b) 1 1.5 and (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. 2. | ||
In the Table 1 it is presented the peak attribution to the main hydrogens signals in the NMR spectra. The amide formation can be observed by the presence of the peaks 2, 4, 5 and 9. It can be observed that the formation of the amide after the thermal treatment occurs in the both EDTA to EDA conditions. Peak 2 and 4 are attribute to the methylene protons of the carbon in the core of the EDTA molecule direct linked to the tertiary amine. Peak 2 is attribute to the EDTA molecules with only one of the two carboxyl in one side of the EDTA converted to amide, while is the peak 4 is attributed to the ETDA molecules with complete converted to amide. The comparison between the intensity of peak 2 and 4 can provide an important understanding of the degree of amide formation on the reaction. It can be observed a severe change in the extend of amide formation between the samples with different EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio. In the sample prepared with the EDTA
EDA ratio. In the sample prepared with the EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio of 1
EDA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, peak 2 and 4 have intensity that are not very distinct. However, the sample prepared with EDTA
1.5, peak 2 and 4 have intensity that are not very distinct. However, the sample prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio of 1
EDA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, peak 4 is much more intense than peak 2. These result indicates content of amide groups formed is much higher in the sample prepared with EDTA
2, peak 4 is much more intense than peak 2. These result indicates content of amide groups formed is much higher in the sample prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio of 1
EDA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The same conclusion can be drawn based in the intensities of peak 5. Peak 5 is the predominant peak in the sample EDTA
2. The same conclusion can be drawn based in the intensities of peak 5. Peak 5 is the predominant peak in the sample EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for the methylene group, which is neighbouring to the carbonyl group in the EDTA. Peak 5 referred to methylene groups neighbor to carbonyl in amide groups while peak 3 is referent to the methylene group neighbor to carbonyls in carboxylic acid. In the sample EDTA
2 for the methylene group, which is neighbouring to the carbonyl group in the EDTA. Peak 5 referred to methylene groups neighbor to carbonyl in amide groups while peak 3 is referent to the methylene group neighbor to carbonyls in carboxylic acid. In the sample EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 the non-modified carbonyl groups are predominant. In the sample EDTA
1.5 the non-modified carbonyl groups are predominant. In the sample EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, peak 5 is a dominant signal while peak 3 is a weak signal.
2, peak 5 is a dominant signal while peak 3 is a weak signal.
In terms of polymerization these molecules are very interesting, EDTA is a tetrafunctional molecule (AB4-type) and EDA is bifunctional molecule (AB2-type), where A and B refer to two reactive functional groups. The NMR results indicate that the stoichiometric sample (EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) had a higher conversion to amide. It suggests that the better organization of the EDTA
2) had a higher conversion to amide. It suggests that the better organization of the EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA crystal provided by the stoichiometric sample induce to a higher polymerization level.
EDA crystal provided by the stoichiometric sample induce to a higher polymerization level.
In the Fig. 4 the 13C NMR spectra of the samples EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 are presented. 13C NMR spectra shows signals of amide C
2 are presented. 13C NMR spectra shows signals of amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the region of δC 178.3–175.6, related to carbons refereed as a in the schematic chemical structure in Fig. 4, confirming the polymerization of the monomers and HBPAs formation. There are signals in δC 182.2–180.0, related to C
O in the region of δC 178.3–175.6, related to carbons refereed as a in the schematic chemical structure in Fig. 4, confirming the polymerization of the monomers and HBPAs formation. There are signals in δC 182.2–180.0, related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of carboxylate terminal groups (b carbons of the chemical structure of Fig. 4). Signals at δC 62.0–60.5 and δC 55.7–54.2 are attributed to the carbons linked to the branching units (c and d carbons, Fig. 4). Finally, carbon directly linked to the aminium terminal groups appear in δC 42.8–42.0 and δC 41.6–40.8 (represented by e and f carbons in Fig. 4).
O of carboxylate terminal groups (b carbons of the chemical structure of Fig. 4). Signals at δC 62.0–60.5 and δC 55.7–54.2 are attributed to the carbons linked to the branching units (c and d carbons, Fig. 4). Finally, carbon directly linked to the aminium terminal groups appear in δC 42.8–42.0 and δC 41.6–40.8 (represented by e and f carbons in Fig. 4).
The molecular weight (Mn) and dispersity index (PDI) of the HBPAs were analysed by GPC in water, Fig. S6 and S7.† HBPAs obtained from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio 1
EDA ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 showed very low PDI of 1.30 and 1.12, respectively. The value obtained for the EDTA
2 showed very low PDI of 1.30 and 1.12, respectively. The value obtained for the EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA is comparable to dendrimers synthesis and it can be considered almost monodisperse. Dispersity values in the range of 1.2 to 1.45 were found for dendritic polymers prepared by multiple steps approach (branch generations).41 The very low dispersity index found for the HBPAs surpass the expectation, since they are produced by a very simple polymerization reaction performed in just one synthesis step. This result indicates that the solid reaction of organized molecular ions can provide great control of molecular mass.
EDA is comparable to dendrimers synthesis and it can be considered almost monodisperse. Dispersity values in the range of 1.2 to 1.45 were found for dendritic polymers prepared by multiple steps approach (branch generations).41 The very low dispersity index found for the HBPAs surpass the expectation, since they are produced by a very simple polymerization reaction performed in just one synthesis step. This result indicates that the solid reaction of organized molecular ions can provide great control of molecular mass.
In addition, in the GPC study it was obtained Mn values of 7.3 kDa for HBPA prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio 1
EDA ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. HBPA obtained from EDTA
1.5. HBPA obtained from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 has molar weight very close to PAMAM G3-NH2 (Mn = 6.9 kDa). A slight lower Mn values was obtained for HBPA from EDTA
1.5 has molar weight very close to PAMAM G3-NH2 (Mn = 6.9 kDa). A slight lower Mn values was obtained for HBPA from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 5.5 kDa. HBPA prepared with EDTA
2 5.5 kDa. HBPA prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio of 1
EDA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 is bigger than HBPA prepared with EDTA
1.5 is bigger than HBPA prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Even though the molecular weight is slightly difference, it has to be considered the NMR results indicate a higher content of the amide groups in the EDTA
2. Even though the molecular weight is slightly difference, it has to be considered the NMR results indicate a higher content of the amide groups in the EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. NMR and GPC results can be correlated to understand a different branching level between the two HBPAs. While the molar weight of the sample from EDTA
2. NMR and GPC results can be correlated to understand a different branching level between the two HBPAs. While the molar weight of the sample from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is lower its amide content is much higher due a greater branching level.
2 is lower its amide content is much higher due a greater branching level.
HBPAs pH-dependent properties were evaluated by DLS and zeta potential. In the Fig. 5 is presented hydrodynamic diameter and zeta potential as a function of pH. A general trend is observed, values of hydrodynamic diameter and zeta potential increase by decreasing pH values.
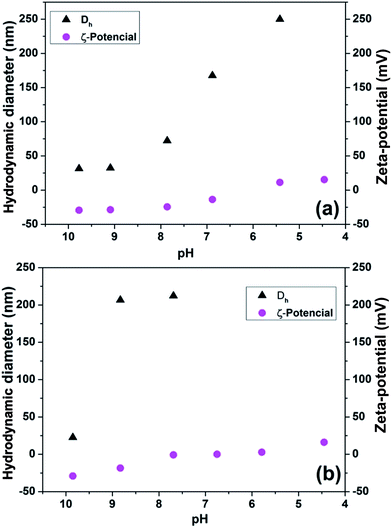 | ||
Fig. 5 Graphics hydrodynamic diameter and zeta potential due to pH for HBPAs synthesized at 160 °C for 2 hours and with EDTA and EDA ratios: (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and (b) 1 1.5, and (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. 2. | ||
The pH-dependence profile of HBPAs shows three distinct regions of protonation in the zeta potential for the HBPA prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA ratio of 1
EDA ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and two regions for HBPA prepared with EDTA
2 and two regions for HBPA prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. It is in agreement with the distinguish pKa values of interior tertiary amine and surface primary amines in dendrimers. In the case of PAMAM, interior tertiary amine groups have pKa around 6.5, while primary amine groups have higher pKa values around 9.2.42,43 In addition, terminal carboxylic acid has pKa around 2.5 and they are prominently deprotonated in the range analysed. Considering that only high branching level is obtained at EDTA
1.5. It is in agreement with the distinguish pKa values of interior tertiary amine and surface primary amines in dendrimers. In the case of PAMAM, interior tertiary amine groups have pKa around 6.5, while primary amine groups have higher pKa values around 9.2.42,43 In addition, terminal carboxylic acid has pKa around 2.5 and they are prominently deprotonated in the range analysed. Considering that only high branching level is obtained at EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA of 1
EDA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the formation of a core with lower pKa value of internal tertiary amine, creates a new transition in the protonation condition. It has to be thought that the increase content of EDA in the sample also generates a higher content of amine terminal groups that form the transition around pH value of 8, that is not observed for HBPA prepared with EDTA
2, the formation of a core with lower pKa value of internal tertiary amine, creates a new transition in the protonation condition. It has to be thought that the increase content of EDA in the sample also generates a higher content of amine terminal groups that form the transition around pH value of 8, that is not observed for HBPA prepared with EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5.
1.5.
In the DLS measurements is observed that the hydrodynamic diameter of both samples are around 25 nm at pH values close to 10. Lowering the pH the polymer particles starts to growth due aggregation of particles caused by the change in the zeta potential. Zeta potential is more negative in higher pH values and it becomes closer to zero as the pH change to higher acidic condition. Lowering the surface charges, the repulsion among the particles became weaker and the van der Waals interactions drive the particle growth. Nevertheless, the ration among EDTA and EDA has an intense effect in the susceptibility to the particle aggregation in function of pH. HBPA from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 rapidly increases its hydrodynamic diameter in pH close to 9, reaching values higher than 200 nm in the pH range of 9 to 7.5. At lower pH values, HBPA from EDTA
2 rapidly increases its hydrodynamic diameter in pH close to 9, reaching values higher than 200 nm in the pH range of 9 to 7.5. At lower pH values, HBPA from EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dispersion becomes unstable due to zeta potential close to zero and the DLS was not able to provide values of hydrodynamic diameter. It occurs because the particle size exceed the maximum particles size measured by the equipment (5 μm). Nonetheless, in the sample prepared by EDTA
2 dispersion becomes unstable due to zeta potential close to zero and the DLS was not able to provide values of hydrodynamic diameter. It occurs because the particle size exceed the maximum particles size measured by the equipment (5 μm). Nonetheless, in the sample prepared by EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EDA 1
EDA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, hydrodynamic diameter is increasing occurs at pH values below 8. In this case the increase in the hydrodynamic diameter follow a linear trend in function of the pH decrease.
1.5, hydrodynamic diameter is increasing occurs at pH values below 8. In this case the increase in the hydrodynamic diameter follow a linear trend in function of the pH decrease.
Conclusions
Solid-state synthesis of hyperbranched polyamides (HBPAs) is demonstrated and the achievement of the polymerized products verified and characterized by FTIR, NMR, GPC, DLS and zeta-potential analyses. Solid-state synthesis demonstrated to be a viable approach to produce dendritic polymer in a one-step process. In addition, the synthesis condition based in the simple thermal treatment of the EDTA/EDA crystals in the temperature range of 120 to 160 °C was shown to be effective to induce the condensation reaction. NMR and GPC results indicates that the EDTA and EDA ratio can be used to change the polymers structure due observed changes in the branching levels. It unfolds many possibilities to the HBPAs synthesized by solid-state procedure since its general properties can be tuned by a simple change in the precursors ratio.Despite the success in the carried out the polymerization synthesis of a HBPAs for the first time by a solid-state reaction, the most important result obtained was the dispersity index of the macromolecules obtained. Dispersity index of 1.3 and 1.12 for the two synthesized HBPAs is similar to the obtained by many steps synthesis of dendrimers. As conclusion, solid-state methodology is an appealing method for dendritic polymers synthesis, since it provides an easy way for large scale synthesis of practically monodisperse polymers.
Acknowledgements
R. S. and A. F. R. acknowledges the financial supports given by CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil), and Fundação Araucária-Brasil. V. A. T. also thanks the CAPES for the graduate fellowships.Notes and references
- R. Silva, M. H. Kunita, E. M. Girotto, E. Radovanovic, E. C. Muniz, G. M. Carvalho and A. F. Rubira, J. Braz. Chem. Soc., 2008, 19, 1224–1229 CrossRef CAS.
- E. Boisselier, A. K. Diallo, L. Salmon, C. Ornelas, J. Ruiz and D. Astruc, J. Am. Chem. Soc., 2010, 132, 2729–2742 CrossRef CAS PubMed.
- R. Guo, Y. Yao, G. Cheng, S. H. Wang, Y. Li, M. Shen, Y. Zhang, J. R. Baker Jr, J. Wang and X. Shi, RSC Adv., 2012, 2, 99–102 RSC.
- D. Astruc, L. Y. Liang, A. Rapakousiou and J. Ruiz, Acc. Chem. Res., 2012, 45, 630–640 CrossRef CAS PubMed.
- S. E. Stiriba, H. Kautz and H. Frey, J. Am. Chem. Soc., 2002, 124, 9698–9699 CrossRef CAS PubMed.
- R. Silva, E. C. Muniz and A. F. Rubira, Appl. Surf. Sci., 2009, 255, 6345–6354 CrossRef CAS.
- W. Liu and C.-M. Dong, Macromolecules, 2010, 43, 8447–8455 CrossRef CAS.
- I. J. Majoros, A. Myc, T. Thomas, C. B. Mehta and J. R. Baker, Biomacromolecules, 2006, 7, 572–579 CrossRef CAS PubMed.
- A. Akesson, M. Cardenas, G. Elia, M. P. Monopoli and K. A. Dawson, RSC Adv., 2012, 2, 11245–11248 RSC.
- L. Zhou, J. L. Geng, G. Wang, J. Liu and B. Liu, ACS Macro Lett., 2012, 1, 927–932 CrossRef CAS.
- R. M. Crooks, M. Q. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS PubMed.
- R. W. J. Scott, O. M. Wilson and R. M. Crooks, J. Phys. Chem. B, 2005, 109, 692–704 CrossRef CAS PubMed.
- S. K. Choi, P. Leroueil, M.-H. Li, A. Desai, H. Zong, A. F. L. Van der Spek and J. R. Baker Jr, Macromolecules, 2011, 44, 4026–4029 CrossRef CAS.
- M. Salem, S. Rohani and E. R. Gillies, RSC Adv., 2014, 4, 10815–10829 RSC.
- R. Silva, G. M. Pereira, E. C. Muniz and A. F. Rubira, Cryst. Growth Des., 2009, 9, 3307–3312 CAS.
- F. Daebritz, B. Voit, M. Naguib and M. Sangermano, Polymer, 2011, 52, 5723–5731 CrossRef CAS.
- J. Khandare, M. Calderon, N. M. Dagia and R. Haag, Chem. Soc. Rev., 2012, 41, 2824–2848 RSC.
- K. L. Wooley, C. J. Hawker and J. M. J. Frechet, Angew. Chem., Int. Ed. Engl., 1994, 33, 82–85 CrossRef.
- K. Inoue, Prog. Polym. Sci., 2000, 25, 453–571 CrossRef CAS.
- D. A. Tomalia, Prog. Polym. Sci., 2005, 30, 294–324 CrossRef CAS.
- R. Silva, E. C. Muniz and A. F. Rubira, Langmuir, 2009, 25, 873–880 CrossRef CAS PubMed.
- D. A. Tomalia, Soft Matter, 2010, 6, 456–474 RSC.
- S. M. Grayson and J. M. J. Frechet, Chem. Rev., 2001, 101, 3819–3867 CrossRef CAS PubMed.
- G. J. Chen, L. Tao, G. Mantovani, V. Ladmiral, D. P. Burt, J. V. Macpherson and D. M. Haddleton, Soft Matter, 2007, 3, 732–739 RSC.
- B. I. Voit and A. Lederer, Chem. Rev., 2009, 109, 5924–5973 CrossRef CAS PubMed.
- S. Li, J. Han and C. Gao, Polym. Chem., 2013, 4, 1774–1787 RSC.
- A. Natarajan, C. K. Tsai, S. I. Khan, P. McCarren, K. N. Houk and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2007, 129, 9846–9847 CrossRef CAS PubMed.
- R. Silva, E. C. Muniz and A. F. Rubira, Polymer, 2008, 49, 4066–4075 CrossRef CAS.
- K. Varga, J. Volaric and H. Vancik, CrystEngComm, 2015, 17, 1434–1438 RSC.
- I. J. Majoros, C. R. Williams, D. A. Tomalia and J. R. Baker Jr, Macromolecules, 2008, 41, 8372–8379 CrossRef CAS PubMed.
- P. Bhattacharya, N. Conroy, A. M. Rao, B. A. Powell, D. A. Ladner and P. C. Ke, RSC Adv., 2012, 2, 7997–8001 RSC.
- K. Liu, Z. Xu and M. Yin, Prog. Polym. Sci., 2015, 46, 25–54 CrossRef CAS.
- M. Labieniec-Watala and C. Watala, J. Pharm. Sci., 2015, 104, 2–14 CrossRef CAS PubMed.
- L. F. Tian, X. Shu and J. Zhu, Adv. Mater., 2007, 19, 4548–4551 CrossRef CAS.
- F. D. R. Amado, C. C. Silveira, L. F. Rodrigues Jr, C. A. Ferreira and A. Meneguzzi, Polim.: Cienc. Tecnol., 2008, 18, 244–248 CrossRef CAS.
- H. Hui, X. D. Fan and Z. L. Cao, Polymer, 2005, 46, 9514–9522 CrossRef.
- K. Y. Cheah, G. S. Howarth, K. A. Bindon, J. A. Kennedy and S. E. P. Bastian, PLoS One, 2014, 9, e98921 Search PubMed.
- M. Liu, C. Xie, H. Pan, J. Pan and W. Lu, J. Chromatogr. A, 2006, 1129, 61–66 CrossRef CAS PubMed.
- Y. Jin, L. Song, D. Wang, F. Qiu, D. Yan, B. Zhu and X. Zhu, Soft Matter, 2012, 8, 10017–10025 RSC.
- R. Silva, G. M. Carvalho, E. C. Muniz, G. J. Vidotti and A. F. Rubira, e-Polym., 2007, 7, 1574–1585 Search PubMed.
- H. E. Rogers, P. Chambon, S. E. R. Auty, F. Y. Hern, A. Owen and S. P. Rannard, Soft Matter, 2015, 11, 7005–7015 RSC.
- M. H. Kleinman, J. H. Flory, D. A. Tomalia and N. J. Turro, J. Phys. Chem. B, 2000, 104, 11472–11479 CrossRef CAS.
- A. Kulczynska, T. Frost and L. D. Margerum, Macromolecules, 2006, 39, 7372–7377 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical characterization of products prepared at different syntheses conditions by FTIR. See DOI: 10.1039/c6ra01023g |
| This journal is © The Royal Society of Chemistry 2016 |