Synthesis of poly(N-(2-hydroxypropyl) methacrylamide) brushes by interface-mediated RAFT polymerization†
Ertan Yildirima,
Dilek Cimena,
Adem Zenginb and
Tuncer Caykara*a
aDepartment of Chemistry, Faculty of Science, Gazi University, 06500, Ankara, Turkey. E-mail: caykara@gazi.edu.tr
bDepartment of Chemical Engineering, Faculty of Engineering, Yuzuncu Yil University, TR-65080, Van, Turkey
First published on 25th April 2016
Abstract
The synthesis of poly(N-(2-hydroxypropyl) methacrylamide) [poly(HPMA)] brushes is reported using an interface-mediated reversible addition–fragmentation chain transfer (RAFT) polymerization on a silicon substrate. To produce a RAFT agent immobilized surface, 4-cyano-4-(propylsulfanylthiocarbonyl)sulfanyl pentanoic acid was reacted with 9-decen-1-ol to form a self-assembled monolayer. Poly(HPMA) brushes and free polymers of varied molecular weights between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 48
100 and 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 were then synthesized by RAFT polymerization. The chemical characterization of the modified surfaces was examined using atomic force microscopy, grazing angle-Fourier transform infrared and X-ray photoelectron spectroscopy, ellipsometry and water contact angle measurements. The average distance between grafting sites (D, nm) and grafting density (σ, chains per nm2) of poly(HPMA) brushes calculated from the dry film thickness (h, nm) and the number-average molecular weight (
500 g mol−1 were then synthesized by RAFT polymerization. The chemical characterization of the modified surfaces was examined using atomic force microscopy, grazing angle-Fourier transform infrared and X-ray photoelectron spectroscopy, ellipsometry and water contact angle measurements. The average distance between grafting sites (D, nm) and grafting density (σ, chains per nm2) of poly(HPMA) brushes calculated from the dry film thickness (h, nm) and the number-average molecular weight (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n) of the free polymers were 1.3 nm and 0.52 chains per nm2, respectively, indicating moderate density polymer brush formation.
n) of the free polymers were 1.3 nm and 0.52 chains per nm2, respectively, indicating moderate density polymer brush formation.
Introduction
Solid surface modification is very important, as it can change surface features considerably and thus control the interaction between materials and their environment. In general, there are two different methods to obtain surface modification of solids with polymers: covalent attachment and physisorption. Covalent immobilization, unlike the physisorption method, can prevent the desorption issue and supply a rigid linkage between the solid surfaces and polymer chains.By reason of its capability to definitely control the grafted chains structure with low-to-high range of grafting densities, in order to modify the solid surface, surface-mediated reversible addition–fragmentation chain transfer (RAFT) polymerization was extensively explored. In this process, there are two general ways to surface-graft polymer chains, including using (1) surface-initiated RAFT polymerization and (2) interface-mediated RAFT polymerization.1–3 In surface-initiated RAFT polymerization, the polymer brush layer is produced in situ from an appropriate surface immobilized initiator.4,5 Surface-initiated RAFT polymerization yields a low grafting density, mainly resulting in recombination of radicals, a low initiator/RAFT ratio, low initiation efficiency, and long initiator half-time.6 Chains anchored to the substrate after polymerization would be shorter than those produced at the start. This chain length variation is not only caused by the late initiation, but also caused by a steric hindrance effect of early chains on the propagation of new chains. This results in a wider molecular weight distribution.7
In the interface-mediated RAFT polymerization, the R or Z group approaches are used to obtain a RAFT agent immobilized solid substrate. Like the “grafting to” method, in the Z group approach, because of the polymer brush layer barrier, propagation of macro radicals in solution leads to a lowered grafting density.8–10 The R-group approach is applied as a “grafting from” approach wherein the solid substrate acts as part of the leaving R-group, and the surface attached macro radicals are responsible for growing the grafted polymer chains on the surface. Because the polymer brushes synthesized by the R group approach have a high grafting density, the Z group approach is not a very common application.10–14
The R group approach can be applied by two different ways on silicon substrates, either by the reaction of the silane coupling agent of the RAFT agent and the silanol sites on a silicon substrate or by covalent immobilization of the inter-linker molecules directly onto a silicon surface via Si–C bonds and subsequent RAFT agent binding in two sequential steps.15–21 The latter approach is preferred because of the thermal and chemical stability (against solvent or acid/base treatments) of the resulting Si–C bonds.22–24 However, to the best of our knowledge, covalent immobilization of 4-cyano-4-(propylsulfanylthiocarbonyl)sulfanyl pentanoic acid (CPP) RAFT agent to the self-assembled monolayer (SAM) of 9-decen-1-ol (DO) on a silicon surface and subsequent interface-mediated RAFT polymerization of N-(2-hydroxypropyl) methacrylamide has not been reported to date.
In this study on extending the applicability of the R group approach, we report the first synthesis of poly(N-(2-hydroxypropyl) methacrylamide) [poly(HPMA)] brushes by interface-mediated RAFT polymerization from a CPP immobilized silicon surface (Si-CPP) (Scheme 1). Although, poly(N-(2-hydroxypropyl) methacrylamide) [poly(HPMA)] is used as a vehicle in drug delivery due to it being water-soluble, uncharged, hydrophilic, and biocompatible with nonimmunogenic properties,25,26 it can be also used for a non-fouling film coating of solid surfaces.
 | ||
| Scheme 1 General procedure for preparing CPP-modified silicon surfaces and subsequent formation of poly(HPMA) brushes by interface-mediated RAFT polymerization. | ||
Experimental section
Materials
All chemicals were used as received unless specified. 4,4′-Azobis(4-cyanopentanoic acid) (ACPA, >97%) was purchased from Sigma. RAFT agent 4-cyano-4-(propylsulfanylthiocarbonyl)sulfanyl pentanoic acid (CPP) and monomer N-(2-hydroxypropyl) methacrylamide (HPMA) were synthesized as-described in the literature (Fig. S1–S6†).27–29 Deionized water (>18.3 MΩ cm) was used in all experiments.Substrate preparation and RAFT agent immobilization
The silicon wafers were sliced into rectangular strips of about 1.0 × 1.5 cm2. To remove surface organic residues, the silicon substrates were initially cleaned in a UV/O3 cleaner (Jelight Company Inc.; Irvine, CA: Model 42) for 5 min. To obtain hydrogen-terminated silicon (Si-H) surfaces, the silicon substrates were washed in 2% HF solution for 5 min and then washed with deionized water and dried with a stream of argon.20 μL of 9-decen-1-ol (DO) was injected onto Si-H surfaces. The surface was placed in an argon purged reaction chamber covered with a quartz window. After irradiation with UV light for 4 h, the chamber was opened and DO modified substrates (Si-DO) were washed with ethanol to remove the residuals and dried with argon.
The Si-DO substrates were immersed into a solution of 40 mg of 4-(dimethylamino) pyridine (DMAP) and 81 mg of N,N′-dicyclohexylcarbodiimide (DCC) in 30 mL of dry dichloromethane, pursued by adding 1.0 g of CPP for preparing the Si-CPP substrates. The reaction was performed for 12 h. The Si-CPP substrates were rinsed with deionized water and ethanol and then dried with argon.
Interface-mediated RAFT polymerization of HPMA
The monomer HPMA (0.01 mol) was polymerized from the RAFT agent immobilized surfaces in a buffer solution (pH = 5.5, 100 mL) in the presence of free RAFT agent CPP (1.3 × 10−2 mmol) and initiator ACPA (1.7 × 10−3 mmol) in a glass reactor. The solution was degassed by three freeze–pump–thaw cycles to remove oxygen. The interface-mediated RAFT polymerization was carried out at 70 °C and 4 mL of polymerization solution were removed with a syringe from time to time to investigate the kinetic behavior of polymer brushes and free polymers in solution.Instrumental techniques
1H-NMR, 13C-NMR spectra were recorded using a Bruker Ultrashield 300 MHz NMR spectrometer. CDCl3 and tetramethylsilane were used as the solvent and internal standard respectively. The dry thicknesses of the brushes were measured in ambient conditions by an Ellipsometry (model DRE, EL X20C) working with a He–Ne laser (λ = 632.8 nm) at an angle of incidence of 75°. The data with a three-layer model (native silicon (refractive index, n = 3.86) + hydrogen terminated silicon layer (n = 1.46) + brush layer (n = 1.46))30 were used for all thickness measurements. The grazing angle-Fourier transform infrared (GA-FTIR) spectra were obtained from dry polymer layers using a Thermo Nicolet 6700 spectrometer equipped with OMNIC software and a grazing angle attachment under continuous purging with liquid nitrogen for 128 sample scans. The SPECS XPS spectrometer equipped with a Mg Kα X-ray source was used for the X-ray Photoelectron Spectroscopy (XPS) measurements. The wettability of the surfaces was assessed by the water contact angle using a goniometer (DSA 100, Krüss) equipped with a microliter syringe using deionized water (4 μL, 18 MΩ cm resistivity) as the wetting liquid. Atomic Force Microscopy (AFM) images were acquired with a Multimode Atomic Force Microscope (Park Systems XE70 SPM Controller LSF-100 HS) as topographical scans in non-tapping mode in air at a scan rate of 1 Hz. Small aliquots taken from polymerization solution were analyzed by aqueous size exclusion chromatography (ASEC) at 25 °C using Ultrahydrogel columns (120, 250, 500 and 100 Å) Waters 2417 Dual λ absorbance detector (λ = 633 nm), and 20% acetonitrile/80% 0.05 M Na2SO4 (aq) as the eluent with a 1.0 mL min−1 flow rate. The dn/dc of poly(HPMA) in the above eluent was determined to be 0.176 mL g−1 at 25 °C (λ = 633 nm). The Breeze software were used to determine the absolute molecular weights and polydispersities of poly(HPMA) chains.Results and discussion
The interface-mediated RAFT polymerization of HPMA was performed on the RAFT agent immobilized silicon surface to obtain poly(HPMA) brushes with a thickness up to 24 nm by controlling the polymerization time. The inter-linker molecule, DO, was first assembled with UV light on Si-H surfaces and followed by reaction of the hydroxyl end groups with CPP RAFT agent in the presence of N-hydroxysuccinimide (NHS) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC). The SAM of DO and the attachment of CPP were verified with GA-FTIR [Fig. 1, (bottom)], XPS [Fig. 1, (top)], AFM [Fig. 2] and water contact angle measurements. The presence of DO on the silicon surface was apparent from the appearance of a broad hydroxyl band at 3000–3350 cm−1 in the GA-FTIR spectrum [Fig. 1, (bottom), spectrum (a)]. XPS analysis of the DO monolayer [Fig. 1, (top), spectrum (a)] confirmed the presence of C 1s and O 1s peaks at 285.6 and 532.9 eV, respectively. CPP attachment was confirmed by the presence of 2230, 1680 and 1072 cm−1 bands, which are assigned to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching, C
N stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and C
O stretching, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretching vibrations, respectively. The wide-scan XPS spectrum of the CPP overlayer [Fig. 1, (top) spectrum (b)] comprised O 1s, N 1s, C 1s and S 2p peaks at 532.4, 398.7, 285.1 and 163.4 eV, respectively. The thickness of the Si-DO and Si-CPP layers were measured by ellipsometry to be 0.9 ± 0.7 nm and 1.3 ± 0.7 nm, respectively.
S stretching vibrations, respectively. The wide-scan XPS spectrum of the CPP overlayer [Fig. 1, (top) spectrum (b)] comprised O 1s, N 1s, C 1s and S 2p peaks at 532.4, 398.7, 285.1 and 163.4 eV, respectively. The thickness of the Si-DO and Si-CPP layers were measured by ellipsometry to be 0.9 ± 0.7 nm and 1.3 ± 0.7 nm, respectively.
The surface morphologies of the silicon substrate after DO and CPP modifications are shown in Fig. 2. The root-mean-square (rms) roughness of the pure silicon wafer cleaned in a UV/O3 cleaner was 0.17 ± 0.03 nm (Fig. S7†). The Si-DO and Si-CPP surfaces are rather uniform and smooth with 0.52 ± 0.08 nm and 0.93 ± 0.14 nm rms roughness values, respectively. The rms values of these surfaces are significantly different from each other. Moreover, the CPP attachment also induced an important alteration in surface wettability characterized by a dramatic increase in the water contact angle from 28.4 ± 0.9° (Si-DO) to 67.3 ± 1.1° (Si-CPP), as shown in the inset images in Fig. 2.
Interface-mediated RAFT polymerization of HPMA was carried out in the presence of the RAFT agent immobilized silicon substrate, free RAFT agent and initiator to produce free poly(HPMA) and the corresponding poly(HPMA) brushes on the silicon substrate surface simultaneously (Scheme 1). The role of the free RAFT agent was to allow an efficient exchange reaction between graft and free polymers to control the polymerization and obtain low polydispersity (Đ) values.31
In a typical polymerization procedure, the molar ratio between monomer, free RAFT agent and initiator was 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125. The polymerization was at 70 °C for different times (1–24 h). During the polymerization, the kinetic plot of ln([M]o/[M]t) versus polymerization time was linear (Fig. 3(a)). The conversion of the HPMA monomer versus time is also linear, indicating that the process of interface-mediated RAFT polymerization was controlled. The symmetric ASEC chromatograms of free poly(HPMA) are given in Fig. 3(b). It shows that there was almost no irreversible termination of the propagating radicals and the interface-mediated RAFT polymerization of HPMA using CPP as chain transfer agent was of living character.
0.125. The polymerization was at 70 °C for different times (1–24 h). During the polymerization, the kinetic plot of ln([M]o/[M]t) versus polymerization time was linear (Fig. 3(a)). The conversion of the HPMA monomer versus time is also linear, indicating that the process of interface-mediated RAFT polymerization was controlled. The symmetric ASEC chromatograms of free poly(HPMA) are given in Fig. 3(b). It shows that there was almost no irreversible termination of the propagating radicals and the interface-mediated RAFT polymerization of HPMA using CPP as chain transfer agent was of living character.
To presume the molecular weights and Đ of the polymer brushes, free polymer in solution was isolated and analyzed. The ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n and Đ values were given in Fig. 3(c). It is clear that
n and Đ values were given in Fig. 3(c). It is clear that ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n increased linearly with the increase of HPMA conversion and the Đ values were narrow, all
n increased linearly with the increase of HPMA conversion and the Đ values were narrow, all ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n values obtained from ASEC were close to the molecular weight values calculated from 1H NMR based on the integral ratio of the peak at 3.8 ppm to the peak at 5.3 ppm, which are listed in Fig. 3(c).
n values obtained from ASEC were close to the molecular weight values calculated from 1H NMR based on the integral ratio of the peak at 3.8 ppm to the peak at 5.3 ppm, which are listed in Fig. 3(c).
The relationship between the thickness (h, nm) of the poly(HPMA) brush and ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n of the corresponding free polymer formed in the solution is shown in Fig. 3(d). A direct proportion relationship was observed between the brush thickness (h = σ
n of the corresponding free polymer formed in the solution is shown in Fig. 3(d). A direct proportion relationship was observed between the brush thickness (h = σ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n/ρNA × 10−21 where σ (chains per nm2) is the grafting density, ρ (1.12 g cm−3) is the density of polymer and NA (6.02 × 1023 mol−1) is Avogadro's number32) and the of
n/ρNA × 10−21 where σ (chains per nm2) is the grafting density, ρ (1.12 g cm−3) is the density of polymer and NA (6.02 × 1023 mol−1) is Avogadro's number32) and the of ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n the free polymer (we assumed that the free polymer and the brush with the same
n the free polymer (we assumed that the free polymer and the brush with the same ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n are formed in the same polymerization solution33,34). From the slope of the linear line (Fig. 3(d)), the grafting density of the poly(HPMA) brush was calculated to be 0.52 chains per nm2, indicating the moderate density polymer brush formation. In this calculation, the average distance between grafting points (D = (4/πσ)1/2)32 was found to be 1.3 nm. The radius of gyration of the poly(HPMA) (
n are formed in the same polymerization solution33,34). From the slope of the linear line (Fig. 3(d)), the grafting density of the poly(HPMA) brush was calculated to be 0.52 chains per nm2, indicating the moderate density polymer brush formation. In this calculation, the average distance between grafting points (D = (4/πσ)1/2)32 was found to be 1.3 nm. The radius of gyration of the poly(HPMA) (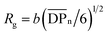 where b is the segment length (assumed to be 0.797 nm for poly(HPMA) chains)) and
where b is the segment length (assumed to be 0.797 nm for poly(HPMA) chains)) and 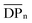 is the degree of polymerization35 was estimated to be 3.2 nm. Most interestingly, comparison of D with Rg of the corresponding free polymer gave in all cases an inferior (D/2Rg = 0.25) ratio indicating the brush-like conformation.
is the degree of polymerization35 was estimated to be 3.2 nm. Most interestingly, comparison of D with Rg of the corresponding free polymer gave in all cases an inferior (D/2Rg = 0.25) ratio indicating the brush-like conformation.
GA-FTIR and XPS measurements were used to characterize the poly(HPMA) brushes. The presence of the poly(HPMA) was confirmed by the prominent amide I and II bands recorded at 1720 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration) and 1560 cm−1 (N–H bending vibrations), respectively (Fig. 4 (top)). Aliphatic C–H bands appeared in the 2850–3000 cm−1 region. Moreover, the intensity of these bands increased with the polymerization time, indicating an increase of poly(HPMA) film thickness. On the other hand, as shown in the XPS spectra (Fig. 4 (bottom)), the intensity of N 1s signals and the decrease of S 2p signals confirm the increase of the film thickness with polymerization time (the thickness of the poly(HPMA) brushes for 5 h and 24 h was 11.3 and 23.6 nm, respectively, and larger than ∼10 nm that is a typical XPS depth36,37).
O stretching vibration) and 1560 cm−1 (N–H bending vibrations), respectively (Fig. 4 (top)). Aliphatic C–H bands appeared in the 2850–3000 cm−1 region. Moreover, the intensity of these bands increased with the polymerization time, indicating an increase of poly(HPMA) film thickness. On the other hand, as shown in the XPS spectra (Fig. 4 (bottom)), the intensity of N 1s signals and the decrease of S 2p signals confirm the increase of the film thickness with polymerization time (the thickness of the poly(HPMA) brushes for 5 h and 24 h was 11.3 and 23.6 nm, respectively, and larger than ∼10 nm that is a typical XPS depth36,37).
Moreover, because of the hydrophilic character of poly(HPMA), the water contact angle decreased from 69.2° ± 0.2° to 45.5° ± 0.1° with increased polymerization time (Fig. 5). The small aggregates were shown on surfaces obtained at shorter polymerization times (Fig. 5). Similar morphologies with closely packed chaines were observed for longer polymerization times. Moreover, it should be noted that variation of rms and h with polymerization time (t) was almost similar (Fig. 6(a)). Moreover, the rms values increased with the increase of h values (Fig. 6(b)). This behavior may be attributed to reorganization of the surface-anchored polymer chains for a more closely packed morphology.
 | ||
| Fig. 6 Evolution of the thickness (h, nm) and rms (nm) of the poly(HPMA) brushes as a function of the polymerization time (t) (a) and variation of rms (nm) with h (nm) (b). | ||
Conclusions
Poly(HPMA) brush fabrication was carried out for the first time from Si-CPP surfaces. The hydrogen-terminated silicon surfaces were obtained by etching the cleaned substrates with HF solution and then DO was immobilized via UV light irradiation on these surfaces to generate the RAFT agent immobilized surfaces. Using these RAFT-CPP modified silicon surfaces, poly(HPMA) brushes with different molecular weights were produced. In each situation, sacrificial CPP was added to polymerization solution to control molecular weight growth from the surface and maintain a “living” mechanism. We achieved a moderate grafting density for the poly(HPMA) brushes (0.52 chains per nm2), and the D/2Rg value was less than 1, indicating that the chains were in a stretched, brush like conformation. We think that the interface-mediated RAFT polymerization from silicon surfaces is particularly hopeful for preparing biosensing and non-fouling materials.Acknowledgements
The authors thank the Scientific and Technical Council of Turkey (TUBITAK), KBAG-114Z319 for its financial support.Notes and references
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2002, 58, 379–410 CrossRef.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2006, 59, 669–692 CrossRef CAS.
- Q. Peng, D. M. Y. Lai, E. T. Kang and K. G. Neoh, Macromolecules, 2006, 39, 5577–5582 CrossRef CAS.
- B. Zaho and W. J. Brittain, Prog. Polym. Sci., 2000, 25, 677–710 CrossRef.
- K. Kato, E. Uchida, E. T. Kang, Y. Uyama and Y. Ikada, Prog. Polym. Sci., 2003, 28, 209–214 CrossRef CAS.
- D. Zhou, E. Mastan and S. Zhu, Macromol. Theory Simul., 2012, 21, 602–614 CrossRef CAS.
- M. Beija, J. D. Martty and M. Destarac, Prog. Polym. Sci., 2011, 36, 845–886 CrossRef CAS.
- P. Takolpuckdee, C. A. Mars and S. Perrier, Org. Lett., 2005, 7, 3449–3452 CrossRef CAS PubMed.
- S. Perrier, P. Takolpuckdee and C. A. Mars, Macromolecules, 2005, 38, 6770–6774 CrossRef CAS.
- L. Barner, C. Li, X. Hao, M. H. Stenzel, C. Barner-Kowollik and T. P. Davis, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5067–5076 CrossRef CAS.
- W. H. Yu, E. T. Kang and K. G. Neoh, Ind. Eng. Chem. Res., 2004, 43, 5194–5202 CrossRef CAS.
- G. Zhai, W. H. Yu, E. T. Kang, K. G. Neoh, C. C. Huang and D. J. Liaw, Ind. Eng. Chem. Res., 2004, 43, 1673–1680 CrossRef CAS.
- C. Yoshikawa, A. Goto, Y. Tsujii, T. Fukuda, K. Yamamoto and A. Kishida, Macromolecules, 2005, 38, 4604–4610 CrossRef CAS.
- G. Pirri, M. Chiari, F. Damin and A. Meo, Anal. Chem., 2006, 78, 3118–3124 CrossRef CAS PubMed.
- A. Zengin, E. Yildirim and T. Caykara, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 954–962 CrossRef CAS.
- N. Zammarelli, M. Luksin, H. Raschke, R. Hergenroder and R. Weberskirch, Langmuir, 2013, 29, 12834–12843 CrossRef CAS PubMed.
- S. Demirci, S. K. Demirci and T. Caykara, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1612–1619 CrossRef CAS.
- D. Cimen, E. Yildirim and T. Caykara, J. Polym. Sci., Part A: Polym. Chem., 2014, 51, 954–962 Search PubMed.
- J. M. Buriak, Chem. Commun., 1999, 1051–1060 RSC.
- R. Boukherroub and D. D. M. Wayner, J. Am. Chem. Soc., 1999, 121, 11513–11515 CrossRef CAS.
- R. L. Cicero, M. R. Linford and C. E. D. Chidsey, Langmuir, 2000, 16, 5688–5695 CrossRef CAS.
- J. M. Buriak, Chem. Rev., 2002, 102, 1272–1308 CrossRef.
- J. M. Buriak, Chem. Commun., 1999, 1051–1060 RSC.
- D. D. M. Wayner and R. A. Wolkow, J. Chem. Soc., Perkin Trans. 2, 2002, 2, 23–24 Search PubMed.
- C. W. Scales, Y. A. Vasilieva, A. J. Convertine, A. B. Lowe and C. L. McCormick, Biomacromolecules, 2005, 6, 1846–1850 CrossRef CAS PubMed.
- M. Vorobii, A. S. Pereira, O. Pop-Georgievski, N. Y. Kostina, C. Rodriguez-Emmenegger and V. Percec, Polym. Chem., 2015, 6, 4210–4220 RSC.
- X. Xu, A. E. Smith, S. E. Kirkland and C. L. McCormick, Macromolecules, 2008, 41, 8429–8435 CrossRef CAS.
- J. Strohalm and J. Kopecek, Angew. Makromol. Chem., 1978, 70, 109–118 CrossRef CAS.
- J. Kopecek and H. Bazilova, Eur. Polym. J., 1973, 9, 7–14 CrossRef CAS.
- K. N. Plunkett, X. Zhu, J. S. Moore and D. E. Leckband, Langmuir, 2006, 22, 4259–4266 CrossRef CAS PubMed.
- Y. Tsujii, M. Ejaz, K. Sato, A. Goto and T. Fukuda, Macromolecules, 2001, 34, 8872–8878 CrossRef CAS.
- I. Luzinov, D. Julthongpiput, H. Malz, J. Pionteck and V. V. Tsukruk, Macromolecules, 2000, 33, 1043–1048 CrossRef CAS.
- E. Turan, S. Demirci and T. Caykara, Thin Solid Films, 2010, 518, 5950–5954 CrossRef CAS.
- M. Baum and W. J. Brittain, Macromolecules, 2002, 35, 610–615 CrossRef CAS.
- S. W. Yeh, K. H. Wei, Y. S. Sun, U. S. Jeng and K. S. Liang, Macromolecules, 2005, 38, 6559–6565 CrossRef CAS.
- L. Li, E. Kang and K. Neoh, Appl. Surf. Sci., 2008, 254, 2600–2606 CrossRef CAS.
- Z. Xu, L. M. Chen, G. Yang, C. H. Huang, J. Hou and Y. Wu, Adv. Funct. Mater., 2009, 19, 1227–1234 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04189b |
| This journal is © The Royal Society of Chemistry 2016 |





