Alkali metal catalyzed dehydro-coupling of boranes and amines leading to the formation of a B–N bond†
A. Harinath,
Srinivas Anga and
Tarun K. Panda *
*
Department of Chemistry, Indian Institute of Technology Hyderabad, Kandi, Sangareddy 502285, Telangana, India. E-mail: tpanda@iith.ac.in; Fax: +91 40 2301 6032; Tel: +91 40 2301 6036
First published on 5th April 2016
Abstract
In this report we describe catalytic B–N bond formation via cross-dehydrocoupling (CDC) of boranes with amines to construct aminoboranes with a high degree conversion (>90%) and chemo-selectivity using alkali metal hexamethyldisilazides [MN(SiMe3)2] (M = Li, and K) as pre-catalysts. It was observed that the lithium and potassium hexamethyldisilazides proved to be an effective pre-catalyst for aliphatic primary, secondary amines, aromatic primary, and substituted amines. The catalyzed cross-dehydrocoupling reaction using [MN(SiMe3)2] (M = Li and K) as a pre-catalyst displayed a broad substrate scope. Pinacolborane smoothly reacted with a number of aliphatic and aromatic amines under ambient conditions whereas a prolonged reaction time of around 8–12 hours was required for 9-BBN to undergo CDC reactions.
Introduction
Green metrics and atom-economical approaches have received significant attention from the communities of organometallic, and organic chemists.1 Recent developments, like increasing costs of raw material, and increased sensitivity to environmental concerns have made atom economical approaches more popular. These approaches can be attained by synthetic efficiency in updating of readily available starting materials to the target products. Thus, the primary focus in these methods are to maximize the incorporation of reactant atoms into the final products.2 To accomplish the target, many researchers restricted their attention on adopting and developing processes those were inherently atom-efficient.3 Over last three decades, the homo- and hetero-dehydrocoupling reaction of E–H (or E′–H) bonds were particularly used to prepare the main group element–element (E–E) bond.4 The cross dehydrogenative coupling (CDC) of N–H and B–H entities has been suggested as an attractive and atom-economical approach to aminoboranes which have valuable chemical applications in potential hydrogen storage.5Historical protocols involving the preparation of aminoboranes are mostly exchange reactions, either with lithium primary amides and B2H6, or of alkali metal hydride and amine boranes.6 Even though some dehydrogenative coupling reactivity exists between protic amines and the parent borane, the synthesis of aminoboranes by this route is impractical and usually requires harsh conditions.7–9 More dependably, the action of tin–nitrogen10 and silicon–nitrogen11 bonds upon boranes and halo boranes yields amino boranes. However, the formation of the group-14 by-products such as tin as toxic waste, is the deficiency of these processes. Subsequently, the most popular synthetic routes to prepare aminoboranes utilize the reaction of lithium amides with BCl3.12 Owing to the above points, a safer and simple dehydrocoupling route to produce aminoboranes by the reaction of hydridic B–H and protic N–H bonds is highly encouraged. Several literature reports are available from last decade, on oligo and polyborazane products which were obtained by the dehydrocoupling of amineboranes adducts RnNH3−n·BH3 (n = 0, 1, 2).13 However, one example of a rhodium-based catalyst14 is known which can catalyze and yield the mono coupled product of an amine and a monohydridoborane when treated with [(HC{(CMe)(N{2,6-iPr2C6H3})}2)Ca(NPh2)(thf)] and 9-BBN.15 Very recently Roesky et al., showed aluminum dihydride LAlH2 (L = HC(CMeNAr)2, Ar = 2,6-Et2C6H3) active catalyst for the dehydro coupling of boranes and amines.16 Recently Hill et al., reported the facile synthesis of aminoboranes from readily available amine and borane precursors wherein alkaline earth metal amides were used as active pre-catalysts.17 In addition, they also reported the dehydrocoupling of Me2NH·BH3 using alkali bis(trimethylsilyl)amide as an active pre-catalyst.18 However, detailed scope of boranes with a wide variety of amines has not been reported till date (Fig. 1).
Mulvey and Robertson recently reviewed the broad utility of various alkali-metal amides.19a The alkali-metal amides represent one of the most commonly encountered classes of reagents in synthetic chemistry today.19b However, in general terms, their continuous application can be accounted for their integrated Brønsted basicity and poor nucleophilicity which place them as competing candidates with alkyllithium reagents which are relatively more basic and yet more nucleophilic in the area of abstracting a proton from a substrate – a pre-requisite for functionalization of a substrate.
Moreover, alkali-metal amides can be mostly dissolved in hydrocarbon media, and are safer to handle than their principal rivals – the alkali-metal hydride or alkyl reagents. In our ongoing work, we have recently developed cross-dehydrocoupling of hydrosilane with amines by using alkali metal amides which are active pre-catalysts.20 On the other hand, the use of alkali amides for the CDC of boranes and amines has not been reported till date. Since these alkali metal amides are easily available, non-toxic and economically viable, we were keen on assessing their use as pre-catalysts in this specific catalyzed reaction. Keeping this in mind, we extended our studies of the group-1 metal amides for the CDC of boranes and amines. We report here the CDC of a wide range of amines with pinacolborane and 9-BBN using LiN(SiMe3)2 and KN(SiMe3)2 as pre-catalysts.
Results and discussion
To begin with, initial screening of the catalytic activity of hexamethyldisilazides [MN(SiMe3)2] (M = Li, Na, K) (Scheme 1) towards CDC of borane with amine was carried out with pyrrolidine and pinacolborane (HBpin), and a catalyst loading of 5 mol%. All three amides proved to be competent catalysts at room temperature and in neat conditions (entries 1, 2 and 3 in Table 1). Near complete conversion was achieved by lithium amide and sodium complex while only 57% conversion was observed for the potassium complex after 1 h (Table 1, entry 3). However, if the reaction time was prolonged to 6 h, it was found that a complete conversion could be achieved. The enhanced solubility of lithium/sodium hexamethylsilazide in the amine contributes to the more rapid conversion of amines vs. the potassium analogue.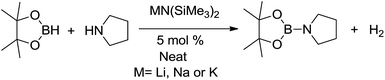 | ||
| Scheme 1 Cross-dehydrogenative coupling (CDC) of HBpin with pyrrolidine mediated by alkali metal complexes [MN(SiMe3)2]. | ||
| Entry | Catalyst | t (h) | Conversion of amine (%) |
|---|---|---|---|
| a General procedure of reaction is as follows: in a glove box, (5 mol%) pre-catalyst was loaded into a Schlenk tube to which amine (1 mmol) and borane (1 mmol) were also added. After the prescribed time, the reaction mixture was transferred into a NMR tube to which 0.6 mL of CDCl3 was added thereafter. Based on the integration of signals in the 1H NMR spectra, conversions on the basis of consumption of amine were obtained.b Reaction mixture was stirred for 6 h. | |||
| 1 | LiN(SiMe3)2 | 1 | 99 |
| 2 | NaN(SiMe3)2 | 1 | 97 |
| 3 | KN(SiMe3)2 | 1, 6b | 57, 99b |
Encouraged by these results, and in these optimal conditions, we studied the scope and generality of the protocol with various amines and substituted anilines with different boranes by using lithium and potassium hexamethyldisilazide as active pre-catalysts anticipating that congeneric sodium salt would show similar activity. The reaction displayed a broad substrate scope. Results for the B–H/H–N CDC are presented in Table 2. In most cases, complete conversion was obtained to afford corresponding aminoboranes using both the pre-catalysts. The weak Lewis acid, HBpin, was observed to couple readily with aliphatic amines of varying bulkiness to yield corresponding amino-boranes in almost complete conversion and within 5 hours at room temperature (entries 1–5). Full substrate conversions were achieved with rapid evolution of hydrogen gas in the case of aliphatic primary amines like nBuNH2 and bulky tBuNH2 (entry 1 and entry 2). Secondary amines like Et2NH, cyclic pyrrolidine and bulky diisopropyl amine underwent 99% conversion at room temperature to yield the corresponding aminoboranes (entries 3, 4 and 5). We thus had an extended substrate scope to aromatic amines. We also observed that, while using both the catalysts at room temperatures, complete conversions occurred at 6–12 h (entry 6–14). The coupling of aniline with pinacolborane in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio yielded the corresponding aminoborane (entry 6) smoothly. However, the benzylamine as coupling partner with pinacolborane produced only 67% of corresponding aminoborane G presumably due to the formation of bis(pinacolato)diboron as byproduct (entry 7 and S5 ESI†). Moreover, the bulky amine DippNH2 did not react with HBpin (entry 8) under similar conditions. Apart from simple anilines, by using our lithium and potassium pre-catalysts, we also investigated those anilines which have the effect of electron withdrawing groups (nitro and halogens) and electron donating groups (Me and OMe), in order to realize their conversion ability with borane.
1 molar ratio yielded the corresponding aminoborane (entry 6) smoothly. However, the benzylamine as coupling partner with pinacolborane produced only 67% of corresponding aminoborane G presumably due to the formation of bis(pinacolato)diboron as byproduct (entry 7 and S5 ESI†). Moreover, the bulky amine DippNH2 did not react with HBpin (entry 8) under similar conditions. Apart from simple anilines, by using our lithium and potassium pre-catalysts, we also investigated those anilines which have the effect of electron withdrawing groups (nitro and halogens) and electron donating groups (Me and OMe), in order to realize their conversion ability with borane.
| Entry | Borane | Amine | Borane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) amine amine |
t (h) | Product | Conv.b (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: [MN(SiMe3)2] (5 mol%), neat reagents (no solvent), room temperature, 12 h (unoptimized reaction time).b Conversions were obtained from integration of signals in the 1H NMR spectra on the basis of consumption of amine.c [LiN(SiMe3)2] as a catalyst.d [KN(SiMe3)2] as a catalyst.e 60 °C. | ||||||
| 1 | HBpin | n-BuNH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5c | 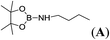 |
99 |
| 8d | ||||||
| 2 | HBpin | tBuNH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 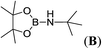 |
99 |
| 3 | HBpin | Et2NH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3c | 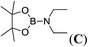 |
99 |
| 6d | ||||||
| 4 | HBpin | 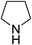 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1c | 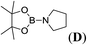 |
99 |
| 6d | ||||||
| 5 | HBpin | [(CH3)2CH]2NH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 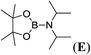 |
99 |
| 6 | HBpin | 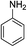 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8c | 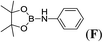 |
99 |
| 8d | ||||||
| 7 | HBpin | Bn-NH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d,e | 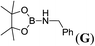 |
67 |
| 8 | HBpin | Dipp-NH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d | 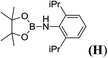 |
0 |
| 9 | HBpin | 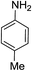 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 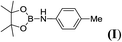 |
99 |
| 10 | HBpin | 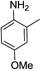 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 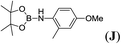 |
95 |
| 11 | HBpin |  |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 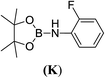 |
99 |
| 12 | HBpin | 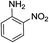 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d | 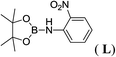 |
50 |
| 13 | HBpin | 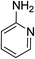 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 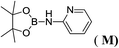 |
86 |
| 14 | HBpin | 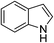 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 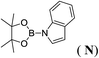 |
99 |
| 15 | HBpin | 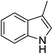 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 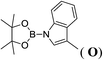 |
45 |
| 16 | HBpin |  |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6d | 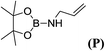 |
90 |
| 17 | 9-BBN | Et2NH | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8d | 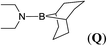 |
99 |
| 18 | 9-BBN | 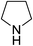 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8d | 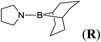 |
99 |
| 19 | 9-BBN | tBuNH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d | 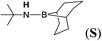 |
99 |
| 20 | 9-BBN | C6H5NH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d | 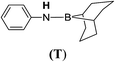 |
50 |
| 21 | 9-BBN | C6H4FNH2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d | 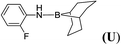 |
32 |
| 22 | HBpin | NH(SiMe3)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12d | 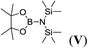 |
0 |
In the case of 4-methylaniline, and 2-methyl-4-methoxy aniline, with pinacolborane as the coupling partner, near complete conversion was achieved (entry 9 and 10) in 6 h. The reaction of 2-fluoro aniline with HBpin too yielded the aminoborane with complete conversion (entry 11). Only 50% conversion was achieved at 12 h (entry 12) when 2-nitroaniline was chosen as the amine substrate to couple with HBpin – this was due to the deactivating nature of the nitro group. In addition to aliphatic or aromatic amines, aromatic heterocyclic amines like 2-amino pyridine and indole were also used as coupling partners. Very good conversion was achieved with respective HBpin and at room temperature in 6 h (entries 13 and 14). We have, in addition, confirmed, by means of single crystal X-ray analysis,21 the solid state structure of coupling product N (Fig. 2) obtained from pinacolborane and indole. However a lower conversion of 45% was obtained for 3-methylindol as coupling partner with pinacolborane (entry 15) and the formation of bis(pinacolato)diboron could be detected as byproduct. It was observed that the alkali metal catalyst was also tolerant of the olefin group as allylamine was easily converted to corresponding aminoborane when treated with pinacolborane (entry 16). The substrate scope was finally extended to bulkier Lewis-acidic 9-BBN. Similar reactions with 9-BBN were also carried out under optimized conditions. Reactions of 9-BBN with primary amine tBuNH2 (entry 19), secondary amine Et2NH (entry 17), and pyrrolidine (entry 18) were sluggish, and they converted to corresponding aminoboranes after a prolonged reaction time of 6–8 h. Conversion of aromatic amine aniline (entry 20) and fluoroaniline (entry 21) to corresponding aminoboranes were achieved at 50% and 32% only respectively when treated for 12 h with 9-BBN. The more bulky amine hexamethyldisilazane showed no conversion with pinacolborane even after 12 h reaction (entry 22). Thus, it can be inferred that steric influences among the substrates are important in order for them to undergo the CDC reaction to form the B–N bond. Similar observations were reported by Hill et al. when HBpin was treated with hexamethyldisilazane using a magnesium based catalyst.16 Thus we can see that the scope of the amine substrates are quite versatile and that they can, in the presence of a lithium or potassium catalyst, easily form a B–N bond. It is to be noted that, in all the cases, a mono-coupled product alone was formed. No di-coupled product was detected even after increasing the borane/amine ratio to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
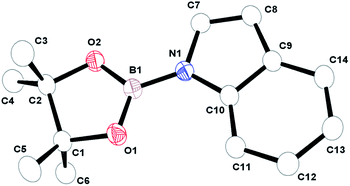 | ||
| Fig. 2 ORTEP drawing of product structure N, the atom labeling scheme; ellipsoids drawn to scale at the 50% probability level. H atoms are omitted for clarity. | ||
Scheme 2 describes a plausible mechanistic pathway for the CDC reaction between organo borane and amines mediated by group-1 hexamethyldisilazido pre-catalysts. This mechanism is based on the recently proposed catalytic cycle for the alkali metal catalyzed cross-dehydrogenative coupling of silane with amine,20 as well as the alkaline-earth promoted catalysis of N–H/H–B CDC reactions.17
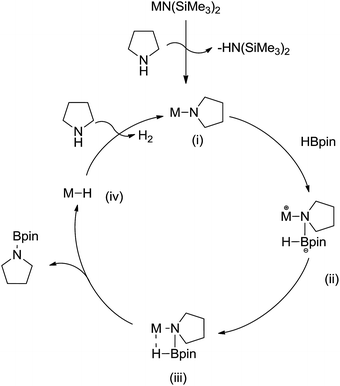 | ||
| Scheme 2 Proposed mechanism for the cross-dehydrogenative coupling of HBpin with pyrrolidine catalyzed by [MN(SiMe3)2] (M = Li, Na, K). | ||
In the initial step, the alkali metal complex, reacts with pyrrolidine to generate a metal pyrrolide (i) via elimination of HN(SiMe3)2, the metal pyrrolide (i) acts as the catalytically active species. In the next step, nucleophilic attack of the Npyrrolide atom onto the electrophilic B center of the incoming borane (illustrated with pinBH in Scheme 2) furnishes the intermediate (ii), and featuring the transient intermediate (iii). However, the transient intermediate (iii) rapidly undergoes β-hydrogen transfer to the metal ion in order to yield the transient metal hydride [MH] (iv) upon release of the coupled aminoborane. In the final step, with elimination of H2, the metal hydride reacts with another molecule of pyrrolidine to regenerate the active metal-pyrrolido species.
Experimental
General
All manipulations of air-sensitive materials were performed under inert atmosphere and in flame-dried Schlenk-type glassware, either on a dual manifold Schlenk line interfaced with a high vacuum (10−4 Torr) line, or in an argon-filled M-BRAUN glovebox. 1H NMR (400 MHz) and 13C{1H} (100 MHz), 11B{1H} (128.2 MHz) spectra were recorded on a BRUKER AVANCE III-400 spectrometer. All amines and boranes were purchased from either Sigma Aldrich or Alfa Aesar. Amines were distilled over CaH2 prior to use. LiN(SiMe3)2, NaN(SiMe3)2 and KN(SiMe3)2 were purchased from Sigma Aldrich and used as received. NMR solvent (CDCl3) was purchased from Alfa Aesar and distilled over molecular sieves.Typical procedure for CDC reactions
All catalytic reactions were performed by using standard protocol as follows, in side the glove box, the chosen precatalyst (0.05 mmol) was added into a Schlenk tube, and subsequently, the amine (n × 0.05 mmol, n equiv.) followed by the borane (n × 0.05 mmol, n equiv.) were added to the Schlenk tube. The Schlenk tube was takeout and stirred in an oil bath at desired temperature (25 °C). After the required period of time, the reaction was quenched by adding CDCl3 to the reaction mixture. Substrate conversion was monitored by examination of the 1H NMR, spectrum of the reaction mixture, comparing relative intensities of resonances characteristic of the substrates and products.Characterization of the products
The data for pinBNHnBu (A), pinBNHtBu (B), pinBNEt2 (C), pinBN(CH2)4 (D), pinBNHC6H5 (F), R2BNHtBu (R) and C6H5NHBR2 (T) are already described in the literature.17 1H, 11B and 13C NMR spectra of aminoboranes, E, G, I, J, K, L, M, N, O, P, Q, R and U are given in the ESI.†Conclusion
To sum up, we have described that easily available, non-toxic and economically viable alkali metal hexamethyldisilazides [MN(SiMe3)2] act as competent pre-catalysts for the cross-dehydrogenative coupling N–H fragment of various amines with of B–H bond of pinacolborane and 9-BBN. Even mostly the pinacolborane could be converted 99%, 9-BBN displayed a poor conversion with different amines. However, benzylamine and 3-methylindol as coupling partners with pinacolborane were converted to respective aminoborane in relatively lower yield. Nevertheless, the lithium and potassium hexamethyldisilazides proved to be an effective pre-catalyst for aliphatic primary amines, aromatic primary, secondary and substituted amines for achieving high conversion and chemoselectivity.Acknowledgements
Ministry of New and Renewable Energy (MNRE), India, is gratefully acknowledged for their financial support under project no. 103/209/2013-NT, dated 29th September, 2014. The instrumental facilities were provided by the Indian Institute of Technology Hyderabad (IITH). A. H. and S. A. thank CSIR for their PhD fellowship. We thank Prof. Kazushi Mashima and Dr Hayato Tsurugi from Osaka University for their generous supports. We thank to the reviewers and editor for their valuable suggestions to improve the manuscript.Notes and references
- B. M. Trost, Science, 1991, 254, 1471 CAS.
- B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259 CrossRef CAS.
- B. M. Trost, Atom economy: a challenge for enhanced synthetic efficiency, in Handbook of green chemistry volume 7: green synthesis, ed. C. J. Li, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2012 Search PubMed.
- (a) D. W. Stephan, Angew. Chem., Int. Ed., 2000, 39, 314 CrossRef; (b) T. J. Clark, K. Lee and I. Manners, Chem. Eur. J., 2006, 12, 8634 CrossRef CAS PubMed; (c) R. Waterman, Curr. Org. Chem., 2008, 12, 1322 CrossRef CAS; (d) R. Waterman, Dalton Trans., 2009, 18 RSC.
- T. Autrey, M. Bowden and A. Karkamkar, Faraday Discuss., 2011, 151, 157 RSC.
- (a) L. D. Schwartz and P. C. Keller, J. Am. Chem. Soc., 1972, 94, 3015 CrossRef CAS; (b) P. C. Keller, J. Am. Chem. Soc., 1974, 96, 3078 CrossRef CAS; (c) P. C. Keller, Inorg. Chem., 1975, 14, 438 CrossRef CAS; (d) P. C. Keller, Inorg. Chem., 1975, 14, 440 CrossRef CAS.
- B. Wrackmeyer, H. E. Maisel and M. Herberhold, J. Organomet. Chem., 2001, 637, 727 CrossRef.
- (a) M. Yalpani, R. Boese and R. Kçster, Chem. Ber., 1990, 123, 1275 CrossRef CAS; (b) M. Yalpani, R. Kçster, R. Boese and W. A. Brett, Angew. Chem., 1990, 102, 318 CrossRef CAS; (c) B. Singaram, Heteroat. Chem., 1992, 3, 245 CrossRef CAS.
- H. B. Zhou, K. W. Nettles, J. B. Bruning, Y. Kim, A. Joachimiak, S. Sharma, K. E. Carlson, F. Stossi, B. S. Katzenellenbogen, G. L. Greene and J. A. Katzenellenbogen, Chem. Biol., 2007, 14, 659 CrossRef CAS PubMed.
- (a) T. Seifert, W. Storch and M. Vosteen, Eur. J. Inorg. Chem., 1998, 1343 CrossRef CAS; (b) T. Gasparis-Ebeling and H. Nçth, Chem. Ber., 1990, 123, 261 CrossRef CAS; (c) H. Noeth, P. Otto and W. Storch, Chem. Ber., 1986, 119, 2517 CrossRef CAS.
- (a) J. F. Janik, C. K. Narula, E. G. Gulliver, E. N. Duesler and R. T. Paine, Inorg. Chem., 1988, 27, 1222 CrossRef CAS; (b) B. Wrackmeyer, B. Schwarze and W. Milius, J. Organomet. Chem., 1995, 489, 201 CrossRef CAS; (c) B. Wrackmeyer, G. Kehr and S. Z. Ali, Z. Naturforsch., B: J. Chem. Sci., 1998, 53, 393 CAS.
- (a) Preparation of tri(dimethylamino)borane: T. Yijun and L. Xiao, CN 101440101, 2009 (b) Novel boronizing agent dimethylamino boronic acid pinacolester as well as synthesis and application of novel boronizing agent dimethylamino boronic acid pinacol ester: G. Zhinong and L. Xiao, CN 102093399, 2011.
- (a) K. Mueller, K. Stark, B. Mueller and W. Arlt, Energy Fuels, 2012, 26, 3691 CrossRef CAS; (b) F. H. Stephens, V. Pons and R. T. Baker, Dalton Trans., 2007, 2613 RSC; (c) N. C. Smythe and J. C. Gordon, Eur. J. Inorg. Chem., 2010, 509 CrossRef CAS; (d) B. Peng and J. Chen, Energy Environ. Sci., 2008, 1, 479 CAS; (e) C. W. Hamilton, R. T. Baker, A. Staubitz and I. Manners, Chem. Soc. Rev., 2009, 38, 279 RSC; (f) Z. Huang and T. Autrey, Energy Environ. Sci., 2012, 5, 9257 RSC; (g) T. Autrey, M. Bowden and A. Karkamkar, Faraday Discuss., 2011, 151, 157 RSC; (h) V. M. Parvanov, G. K. Schenter, N. J. Hess, L. L. Daemen, M. Hartl, A. C. Stowe, D. M. Camaioni and T. Autrey, Dalton Trans., 2008, 4514 RSC; (i) A. Staubitz, A. P. M. Robertson and I. Manners, Chem. Rev., 2010, 110, 4079 CrossRef CAS PubMed.
- C. M. Vogels, P. E. OÏConnor, T. E. Phillips, K. J. Watson, M. P. Shaver, P. G. Hayes and S. A. Westcott, Can. J. Chem., 2001, 79, 1898 CrossRef CAS.
- A. G. M. Barrett, M. R. Crimmin, M. S. Hill, P. B. Hitchcock and P. A. Procopiou, Organometallics, 2007, 26, 4076 CrossRef CAS.
- Z. Yang, M. Zhong, X. Ma, K. Nijesh, S. De, P. Parameswaran and H. W. Roesky, J. Am. Chem. Soc., 2016, 138, 2548 CrossRef CAS PubMed.
- D. J. Liptrot, M. S. Hill, M. F. Mahon and A. S. S. Wilson, Angew. Chem., Int. Ed., 2015, 54, 13362 CrossRef CAS PubMed.
- P. Bellham, M. S. Hill and G. Kociok-Köhn, Dalton Trans., 2015, 44, 12078 RSC.
- (a) R. E. Mulvey and S. D. Robertson, Angew. Chem., Int. Ed., 2013, 52, 11470 CrossRef CAS PubMed; (b) M. Lappert, P. Power, A. Protchenko and A. Seeber, Metal Amide Chemistry, Wiley, Chichester, 2009, p. 7 Search PubMed.
- S. Anga, Y. Sarazin, J. F. Carpentier and T. K. Panda, ChemCatChem, 2016, 8, 1373 CrossRef CAS.
- Crystal data for N (CCDC no. 1451650†): C14H18BNO2, FW 243.10, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.904(2) Å, b = 10.603(2) Å, c = 13.511(3) Å, α = 71.18(2)°, β = 88.01(2)°, γ = 88.04(2)°, V = 1341.7(5) Å3, T = 150 K, λ = 1.54184 Å, Z = 4, Dcalcd = 1.203 g cm−3, 2θmax = 71.744°, μ = 0.624 mm−1, R1 and wR2 = 0.11 and 0.38 (I > 2σ(I)), GOF = 1.13.
, a = 9.904(2) Å, b = 10.603(2) Å, c = 13.511(3) Å, α = 71.18(2)°, β = 88.01(2)°, γ = 88.04(2)°, V = 1341.7(5) Å3, T = 150 K, λ = 1.54184 Å, Z = 4, Dcalcd = 1.203 g cm−3, 2θmax = 71.744°, μ = 0.624 mm−1, R1 and wR2 = 0.11 and 0.38 (I > 2σ(I)), GOF = 1.13.
Footnote |
| † Electronic supplementary information (ESI) available: Text giving experimental details for the catalytic reactions, 1H, 13C{1H} and 11B{1H} spectra of aminoboranes E, G, I, J, K, L, M, N, O, P, Q, R and U. CCDC 1451650. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra04125f |
| This journal is © The Royal Society of Chemistry 2016 |

