Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two-photon FRET pairs based on coumarin and DBD dyes†
P.
Wessig
*,
N.
Behrends
,
M. U.
Kumke
,
U.
Eisold
,
T.
Meiling
and
C.
Hille
Institut für Chemie, Universität Potsdam, Karl-Liebknecht-Str. 24-25, 14476 Potsdam, Germany. E-mail: wessig@uni-potsdam.de; Fax: +49-331977-5065; Tel: +49-331977-5401
First published on 29th March 2016
Abstract
The synthesis and photophysical properties of two new FRET pairs based on coumarin as a donor and DBD dye as an acceptor are described. The introduction of a bromo atom dramatically increases the two-photon excitation (2PE) cross section providing a 2PE-FRET system, which is also suitable for 2PE-FLIM.
Amongst various analytical techniques, the evaluation of fluorescence signals is one of the most sensitive methods.1 Therefore, fluorescence spectroscopy has found a wide range of applications in biology, biochemistry, and clinical diagnostics. Besides classic steady state measurements that evaluate the fluorescence intensity, methods utilizing the fluorescence lifetime have gained growing interest in recent years. Both parameters are successfully applied in fluorescence imaging. The pivotal element of these methods is the fluorescent dye and its properties. Small fluorescent organic molecules play, beside quantum dots (QD)2 and luminescent lanthanide complexes,3 the central role in current fluorescence spectroscopy. The potential of a fluorescent dye is determined by the combination of its photophysical parameters, such as fluorescence quantum yield (ΦF), molar extinction coefficient (ε), fluorescence lifetime (τF), absorption and emission wavelength (λABS, λEM), and stability against photobleaching. Because the penetration depth of light in biological tissue is increased with increasing wavelength, high λABS and λEM are desirable. However, a long-wavelength absorption maximum is usually connected with low thermal and photochemical stability of the dyes. This problem can be circumvented by two-photon excitation (2PE),4 which is based on the simultaneous absorption of two photons with lower energy instead of one photon with high energy. Consequently, the excitation wavelength λEXC is almost doubled in 2PE. The probability of the 2PE is described by its 2PE cross section σ2. Unfortunately, σ2 is low for most organic molecules.5 On the other hand the applicability of many fluorescence dyes suitable for 2PE is limited when considering the other photophysical properties. The combination of the outstanding properties of two fluorescent dyes within one molecule can be achieved by a pair of dyes capable for Förster Resonance Energy Transfer (FRET).6 This phenomenon, in which one dye (the donor D) is excited and the energy is transferred to the other dye (the acceptor A), is observed if the emission spectrum of D and the absorption spectrum of A sufficiently overlap. Furthermore, the FRET efficiency is strongly distance-dependent (the distance with 50% FRET efficiency is called Förster distance R0) and is influenced by the relative arrangement of the transition dipole moments of D and A. A special type of FRET exists if the donor D is capable of 2PE. In this case, the fluorescence of the donor D as well as the acceptor A can be observed at shorter wavelength than the excitation (“Anti-Stokes shift”). Although some examples of 2PE-FRET pairs were reported in the literature,7 the number of 2PE-FRET systems is rather limited.
In the last few years, we have developed a new class of fluorescent dyes, whose structure is based on [1,3]dioxolo[4,5-f][1,3]benzo-dioxole (DBD).8–14 These dyes are characterised by large Stokes shifts (Δλ = λEM − λABS > 100 nm), combined with long fluorescent lifetimes (τF > 20 ns) and exceptional bleaching stability. The 2PE cross-sections σ2 of DBD dyes are, however, rather low (vide infra). Recently, we reported on a first FRET pair with DBD dyes as acceptor and 2,5-diphenyloxazol (PPO) as donor.13 Herein we wish to report on the synthesis and properties of new FRET pairs with coumarin derivatives as donor and DBD dyes as acceptor for 2PE-FRET application.
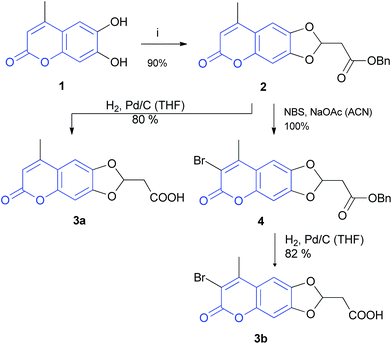
The coumarin chromophore was chosen because it is already known to be suitable for 2PE.15 The synthesis starts with the commercially available 6,7-dihydroxy-coumarin 1. The reaction with benzyl prop-2-ynoate16 in the presence of catalytical amounts of DMAP afforded coumarin 2 with very good yield.
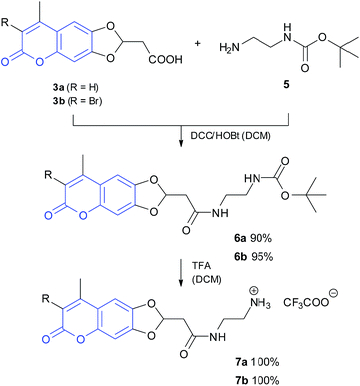
This type of cyclisation has already been described for other catechols8,10,17 but never applied to coumarins. Because the introduction of halogen atoms regularly increases the 2PE cross-section, 2 was brominated with NBS to give 3-bromo-coumarin in quantitative yield. Subsequently, esters 2 and 4 were deprotected by catalytic hydrogenation to the carboxylic acids 3a,b (Scheme 1). The connection between coumarin and DBD chromophore was accomplished by a 1,2-diaminoethane linker. For this purpose, esters 3a,b were converted into amides 6a,b by reaction with commercially available N-Boc-1,2-diaminoethane 5, which were subsequently deprotected to give primary amines 7 (Scheme 2).
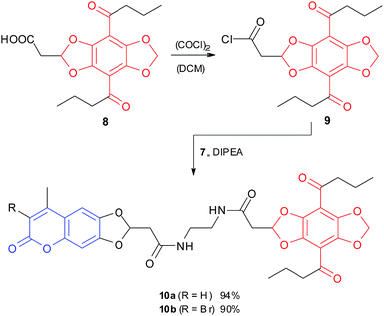
Finally, the FRET pairs 10a,b were prepared from amines 7a,b and acyl chloride 9, which is easily accessible from the known DBD acid 8 (Scheme 3).10
Next, we investigated the spectroscopic properties of compounds 10a,b in comparison with the starting compounds 2, 4, 8. The results in DMSO are summarized in Table 1 (for other solvents see ESI†). Of particular note are the large Stokes shift (133 nm) and the long fluorescence lifetime (24.8 ns) of DBD dye 8 compared with coumarins 2 and 4. The successful FRET in compound 10a,b was shown by 3D-fluorescence experiments, which are exemplarily outlined for 10a in Fig. 1.
| Compound | λ ABS/nm | λ EM/nm | τ F/ns | ε/M−1 cm−1 | Φ F | σ 2/GMa |
|---|---|---|---|---|---|---|
| a Excited at 780 nm (2, 8, 10a,b) or 720 nm (4), GM = 10−50 cm4 per s per photon (threefold measure). b Fluorescence lifetime was detected at 420 nm (λEXC = 375 nm). c Fluorescence lifetime was detected at 570 nm (λEXC = 440 nm). d Fluorescence lifetime was detected at 570 nm (λEXC = 372 nm). | ||||||
| 2 | 343 | 413 | 0.8b | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878 878 |
0.23 | 0.01 ± 17% |
| 4 | 353 | 436 | 1.3b | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 269 269 |
0.17 | 13.86 ± 4% |
| 8 | 437 | 570 | 24.8c | 3094 | 0.68 | 0.04 ± 3% |
| 10a | 344, 433 | 570 | 24.0d | — | — | — |
| 10b | 355, 434 | 570 | 23.5d | — | — | — |
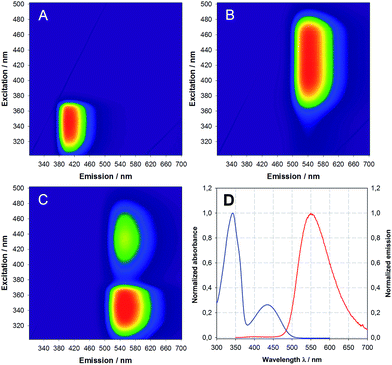 | ||
| Fig. 1 3D excitation emission matrix (EEM) of (A) coumarin 2, (B) DBD 8, (C) FRET pair 10a and (D) absorption (blue) and emission (red) spectra of FRET pair 10a in DCM (excitation at 340 nm). | ||
The highly efficient FRET in compound 10a is stressed in Fig. 1C by (i) the strong quenching of the donor emission (compare to Fig. 1A) and (ii) the strong emission of the DBD acceptor under indirect excitation via the coumarin donor (compare to Fig. 1B). This result was to be expected, because the maximum possible distance between the chromophores (1.5–1.6 nm) is markedly below the Förster radius R0 for this FRET pair (2.56 nm, for details see the ESI†).
Whereas the 2PE cross section σ2 is very low for DBD compound 8 and coumarin 2, the introduction of bromine in coumarin 4 significantly enhances the σ2 value to 0.74 GM (see Table 1).
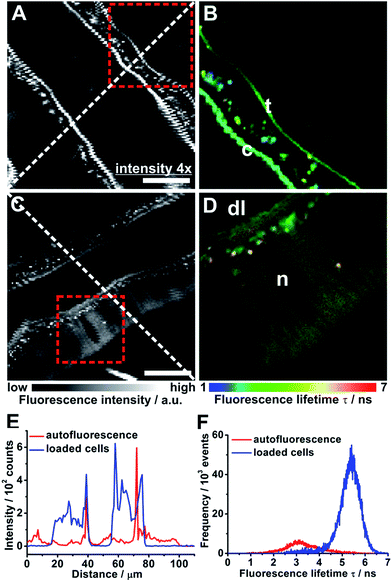
In order to explore potential applications of the 2PE-FRET pairs-sensor 10b in living cells, its uptake into such was studied in 2PE-fluorescence lifetime imaging (2PE-FLIM) experiments. Insect salivary gland lobes were incubated for 15 min with 2 μM 10b and then, the tubular-like salivary ducts were imaged. Salivary ducts without 10b-loading displayed a comparatively low autofluorescence when excited at 780 nm as expected for 2PE (Fig. 2A and E).18 Mainly the luminal cuticule and tracheae contributed to the autofluorescence (Fig. 2B) and the corresponding fluorescence lifetime distribution fluctuated around 3 ns (Fig. 2F). In contrast, the fluorescence intensity of 10b-loaded salivary ducts was up to one order of magnitude higher than that of unloaded ducts (Fig. 2C and E). Such a loading efficiency has previously been reported for other ion-sensitive fluorescent dyes.19 Here, the duct cells were stained strongly and 10b did not accumulate in specific cellular compartments. However, almost no fluorescence could be observed in the nuclei and the duct lumen (Fig. 2D). This result is a prerequisite for successful intracellular recordings using the novel 2PE-FRET pairs presented. The sufficient 10b-loading into living cells could also be observed in the fluorescence lifetime distribution, which was now shifted to longer lifetimes around 5.5 ns (Fig. 2F).
Conclusions
Both single chromophores exhibit specific pros and cons. The 3-bromocoumarin shows a sufficiently large 2PE cross section (0.74 GM) but has a low fluorescence lifetime (1.3 ns) and a relatively short emission wavelength (436 nm).By contrast, the DBD chromophore shows long emission wavelength (570 nm), large fluorescence lifetime (23–25 ns) but very low 2PE cross section. The FRET pair 10b perfectly combines the advantages of these dyes. After long-wavelength two-photon excitation at 780 nm an efficient FRET takes place resulting in a long-lived emission at 570 nm. The applicability of 10b for 2PE-FLIM was demonstrated with the aid of living cells of insect salivary gland lobes. In this application the large contrast range of 1–7 ns is noteworthy. Currently, we are investigating synthetic routes to derivatives of 10b, which are suitable for coupling with various biomolecules.
Notes and references
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 3rd edn, 2006 Search PubMed.
- D. Bera, L. Qian, T.-K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260 CrossRef CAS.
- S. Faulkner, S. J. A. Pope and B. P. Burton-Pye, Appl. Spectrosc. Rev., 2005, 40, 1 CrossRef CAS.
- (a) M. Goeppert-Mayer, Ann. Phys., 1931, 9, 273 CrossRef; (b) B. Valeur, M. N. Berberan-Santos, Molecular Fluorescence, Wiley-VCH, 2nd edn, 2013 Search PubMed; (c) J. S. Park, J.-K. Park, P. Prabhakaran and K.-S. Lee, Nonlinear Opt., Quantum Opt., 2015, 46, 227 Search PubMed.
- N. S. Makarov, M. Drobizhev and A. Rebane, Opt. Express, 2008, 16, 4029 CrossRef CAS PubMed.
- (a) B. Valeur, M. Berberan-Santos, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, 2nd edn, 2012, pp. 213–261 Search PubMed; (b) C. G. dos Remedios and P. D. J. Moens, J. Struct. Biol., 1995, 115, 175 CrossRef CAS PubMed.
- (a) M. Elangovan, H. Wallrabe, Y. Chen, R. N. Day, M. Barroso and A. Periasamy, Methods, 2003, 29, 58 CrossRef CAS PubMed; (b) L. Liu, G. Wei, Z. Liu, Z. He, S. Xiao and Q. Wang, Bioconjugate Chem., 2008, 19, 574 CrossRef CAS PubMed; (c) L. Liu, H. Li, T. Qiu, G. Zhou, K.-Y. Wong, Z. He and Z. Liu, Chem. Commun., 2011, 47, 2622 RSC.
- P. Wessig, R. Wawrzinek, K. Möllnitz, E. Feldbusch and U. Schilde, Tetrahedron Lett., 2011, 52, 6192 CrossRef CAS.
- R. Wawrzinek, P. Wessig, K. Möllnitz, J. Nikolaus, R. Schwarzer, P. Müller and A. Herrmann, Bioorg. Med. Chem. Lett., 2012, 5367 CrossRef CAS PubMed.
- R. Wawrzinek, J. Ziomkowska, J. Heuveling, M. Mertens, A. Herrmann, E. Schneider and P. Wessig, Chem.–Eur. J., 2013, 19, 17349 CrossRef CAS PubMed.
- J. Heuveling, V. Frochaux, J. Ziomkowska, R. Wawrzinek, P. Wessig, A. Herrmann and E. Schneider, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 106 CrossRef CAS PubMed.
- C. Meyners, R. Wawrzinek, A. Krämer, S. Hinz, P. Wessig and F.-J. Meyer-Almes, Anal. Bioanal. Chem., 2014, 4889 CrossRef CAS PubMed.
- R. Wawrzinek and P. Wessig, Dyes Pigm., 2015, 123, 39 CrossRef CAS.
- D. Bader, D. T. Klier, C. Hettrich, F. F. Bier and P. Wessig, Anal. Methods, 2016, 9, 1235 RSC.
- (a) T. Furuta, S. S.-H. Wang, J. L. Dantzker, T. M. Dore, W. J. Bybee, E. M. Calaway, W. Denk and R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1193 CrossRef CAS PubMed; (b) N. S. Makarov, M. Drobishev and A. Rebane, Opt. Express, 2008, 16, 4029 CrossRef CAS PubMed.
- Y. C. Fan and O. Kwon, Org. Lett., 2012, 14, 3264 CrossRef CAS PubMed.
- (a) X. Ariza, O. Pineda, J. Vilarrasa, G. W. Shipps Jr., Y. Ma and X. Dai, Org. Lett., 2001, 3, 1399 CrossRef CAS PubMed; (b) I. Yavari, L. Azad and T. Sanaeishoar, Monatsh. Chem., 2011, 142, 643 CrossRef CAS; (c) I. Yavari, S. Souri, M. Sirouspour and H. Djahaniani, Synthesis, 2006, 19, 3243 CrossRef; (d) C. Chalumeau, D. Deffieux, S. Chaignepain and S. Quideau, ChemBioChem, 2011, 12, 1193 CrossRef CAS PubMed; (e) C. Fu, W. Chen, Y. L. Quek, R. Ni, A. B. A. Ghani, W. W. Y. Leong, H. Zeng and D. Huang, Tetrahedron Lett., 2010, 51, 6322 CrossRef CAS.
- M. Lahn, C. Dosche and C. Hille, Am. J. Physiol.: Cell Physiol., 2011, 300, C1323 CrossRef CAS PubMed.
- (a) K. Sagolla, H.-G. Löhmannsröben and C. Hille, Anal. Bioanal. Chem., 2013, 405, 8525 CrossRef CAS PubMed; (b) K. Jahn and C. Hille, PLoS One, 2014, 9, e105334 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03983a |
| This journal is © The Royal Society of Chemistry 2016 |