Synthesis of CuO/ZnO/Al2O3/ZrO2/CeO2 nanocatalysts via homogeneous precipitation and combustion methods used in methanol steam reforming for fuel cell grade hydrogen production†
Abstract
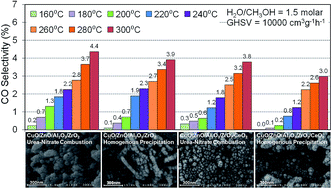
Homogeneous precipitation and urea-nitrate combustion methods have been comparatively investigated for the synthesis of CeO2 promoted CuO/ZnO/Al2O3/ZrO2 nanocatalysts. The catalytic performance of the samples was studied in the methanol steam reforming process for fuel cell grade hydrogen production. The physicochemical properties of the prepared nanocatalysts were characterized by XRD, FESEM, PSD, EDX, BET and FTIR analysis. XRD analysis shows that the homogeneous precipitation method and CeO2 addition improves the dispersion and decreases the relative crystallinity of CuO and ZnO species. FESEM images illustrate that the homogeneous precipitation method is more capable of the synthesis of smaller particles than the urea-nitrate combustion method. Addition of ceria to the fabricated catalysts decreases the particle size and enhances surface homogeneity. EDX analysis demonstrates that Cu and Zn elements are homogeneously dispersed on the catalyst surface, which is synthesized by a homogeneous precipitation method. The catalytic performance demonstrates that applying the homogeneous precipitation method improves activity while the ceria addition leads to lower CO selectivity.

 Please wait while we load your content...
Please wait while we load your content...