Expeditious synthesis of the tetrasaccharide cap domain of the Leishmania donovani lipophosphoglycan using one-pot glycosylation reactions†
Abstract
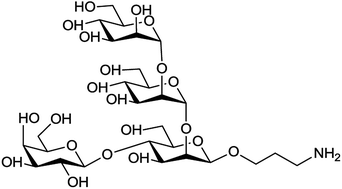
Expeditious syntheses of the tetrasaccharide cap related to the lipophosphoglycan of Leishmania donovani from two monosaccharides and a disaccharide building block were achieved by one-pot sequential glycosylation reactions. The monosaccharide building blocks were synthesized from D-mannose, and the disaccharide building block was prepared from lactose by C2-epimerisation of its glucose unit through C2 hydroxy oxidation–reduction.

 Please wait while we load your content...
Please wait while we load your content...