Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-step, solvent-free mechanosynthesis of silver nanoparticle-infused lignin composites for use as highly active multidrug resistant antibacterial filters†
Monika J.
Rak
,
Tomislav
Friščić
and
Audrey
Moores
 *
*
Centre for Green Chemistry and Catalysis, Department of Chemistry, McGill University, 801 Sherbrooke St. W., Montréal, Québec H3A 0B8, Canada. E-mail: audrey.moores@mcgill.ca; Fax: +1 514 398 3797; Tel: +1 514 398 4654
First published on 8th June 2016
Abstract
Silver nanoparticles were synthesized in a solvent-free fashion from a simple silver salt by ball milling mechanochemistry, using lignin as a biodegradable reducer and polyacrylamide polymer as a support. The resulting material proved very efficient as an antimicrobial filter for complete killing of both Gram positive and Gram negative bacteria, including multi-drug resistant strains.
Introduction
Essential to life, drinkable, clean water is still considered an unaffordable luxury in many parts of the world. The World Health Organization (WHO) estimates that in 2004 around 2 million deaths occurred due to poor water sanitation and hygiene.1 The use of silver for improving water quality has been known since antiquity, and remained in application in Europe until the 1940s,2 when antibiotics successfully supplanted its use.3 However the rise of multidrug resistance in bacteria in the past decades4 has triggered a renewed interest in using silver in antibacterial applications.5–9 The WHO does not recommend a maximum allowed concentration of silver in water,10 reflecting the relative safety of this method and opening the possibility to use silver filters for providing safe drinking water to underprivileged peoples in third world countries.11,12Among silver-based antibacterial materials, silver nanoparticles (AgNPs) are intensely researched and exhibit superior activity because of their high surface-over-volume ratio.3,13 It was shown by Alvarez et al. that the high antimicrobial activity of AgNPs is due to aerobic formation of an oxide layer (Ag2O) which releases antimicrobially-active silver ions in contact with water.14 Several types of AgNP-based filters for point-of-use water treatment have already been developed.15–18 These systems range from simple blotter paper used as AgNPs support15 to polyurethane foams17 and ceramics containing AgNPs. The latter demonstrate particular potential in studies focusing on rural areas of developing countries.19,20 Such filter systems are typically produced using classic approaches for the synthesis of nanomaterials, and are thus burdened by the need for large solvent amounts, extensive use of aggressive reducing agents (e.g. NaBH4) and/or energy intensive thermal treatment. Besides making the production of nanomaterial-based systems unsustainable, such conventional methods also adversely affect the potential for their scale-up.21–23
The production of AgNPs has been the focus of recent efforts to develop greener and increasingly sustainable synthetic approaches to nanomaterials,24e.g. by introducing mild bio-based reducers such as citrate, ascorbate, polysaccharides, polyphenols and lignin.25–31 These methods, however, often rely on high dilution, necessitating extensive solvent use and additional workup steps. In contrast, one-pot approaches which avoid post-treatments, and in which all mixed components are essential to the application, offer an opportunity for nanomaterials production to embrace the concept of atom economy.32,33 Very recently, several examples of such one-pot approaches were reported. For instance, John and coworkers produced AgNPs directly within the intended medium of application, i.e. a commercial oil-based paint.34 Our group has recently showcased the production of AgNPs directly from bulk silver, using cellulose nanocrystals as a support and reducer, in a water suspension used as a medium for catalytic biphasic separation.35 The group of Pradeep later used a similar strategy to extract silver using glucose.36 Also, we have recently demonstrated a mechanochemical approach to generate noble metal (Au, Rh, Ru) nanoparticles that uses lignin both as a reductant and as a support for the resulting NPs.37 Lignin is generated on a multi-ton scale as a by-product of paper manufacturing.38,39 It is largely underutilized and therefore, represents an abundant, inexpensive, and non-toxic renewable resource.40,41 The poor solubility of lignin, due to its highly cross-linked polymeric structure, hinders its extensive use beyond a few successful commercial applications, e.g. in wood panels, epoxy resins, polyurethane foams, biodispersants and automobile brakes.39,40 For the production of solution-based AgNPs, it called for strong basic conditions, and only incomplete functionalization.29,30
In contrast to conventional solution-based reactivity and processing, mechanochemistry by milling or grinding42 provides an opportunity to conduct reactions of poorly soluble substances. Recent in situ studies reveal that rapid mechanical agitation in a conventional laboratory mill enables reactions of poorly soluble substances at rates and kinetics resembling those typically observed in solution chemistry. Milling has already been demonstrated as a versatile technique for the rapid and environmentally-friendly synthesis of nanomaterials through top-down or bottom-up approaches,37,43,44 as well for lignin activation45via mechanical cleavage forming radicals with highly reductive potential.46,47
Herein we report the direct, one-pot and solvent-free synthesis of AgNPs supported within a matrix of lignin and polyacrylamide, leading to stable, inexpensive and readily manufactured composites. These composite materials can be used without workup as antibacterial water filters, as demonstrated herein by their effectiveness against several strains of Gram +ve and Gram −ve bacteria. The presented mechanochemical process is the first example of the direct synthesis of a silver-based filter material directly from AgNO3, without any solvent, harsh reducing agent or post-treatment. The composite materials were fully characterized by transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (PXRD) and Fourier-transform infra-red attenuated total reflectance (FTIR-ATR) spectroscopy. Importantly, silver leaching was monitored by inductively coupled plasma optical emission spectrometry (ICP-OES) and shown to be below 0.31 ppm.
Results and discussion
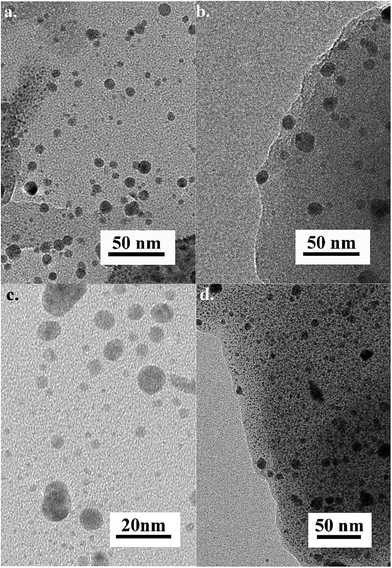
As the first entry into using lignin for mechanochemical formation of AgNPs, we chose AgNO3 as an inexpensive and readily available silver source, in combination with two different types of lignin: commercially available (kraft) and laboratory produced (thermomechanical pulp, TMP) one. The kraft pine lignin is obtained as a waste product of the kraft pulping process which chemically treats wood to achieve separation of lignin from cellulosic components.48 The TMP lignin is derived from a process that relies on exposing the pulp to milling under steam in order to effect the separation from cellulose. It is on average larger in fiber size than kraft lignin and is often called natural lignin as its structure resembles the original lignin structure to a higher extent than that of kraft lignin.49In a typical experiment, solid AgNO3 (50 mg) and lignin (160 mg) were placed into a 10 mL stainless steel milling jar along with two 7 mm stainless steel balls (1.3 grams each) and milled for 90 min using a Retsch MM400 mill operating at 30 Hz (Scheme 1). The resulting solid was scraped off the jar walls and stored at room temperature. The material was washed with water prior to characterization and use. The materials generated using kraft lignin and TMP lignin were named AgNPs@KLig and AgNPs@TMPLig, respectively. TEM analysis of these materials demonstrated the successful formation of AgNPs as dark spots (Fig. 1 and S1–S4†). Both samples featured polydisperse NPs, with particle sizes ranging between 1 nm and 30 nm. TEM also allow to visualize the organic lignin matrix as a lower density material, covering large sections of the sample grids. Interestingly, AgNPs were observed within, as well as outside the lignin matrix, indicating that AgNPs could be easily washed away.
In the prospect of mechanochemically preparing composites containing AgNPs and using them as solid antibacterial filters, we had to introduce a third component, namely polyacrylamide (PAM), as a supporting polymer into the milling process, alongside lignin and silver precursor. PAM is often used in cosmetic formulations and was therefore deemed safe to use in this application.50 A mixture of lignin (either kraft or TMP) and PAM was milled with AgNO3 for 90 min at 30 Hz. The TEM images of the obtained materials revealed the successful formation of composites with AgNPs fully encased in the organic matrix using both kraft lignin (AgNP@KLig/PAM) and TMP lignin (AgNP@TMPLig/PAM), respectively (Fig. 1). In both composites, AgNPs were spherical and polydisperse, exhibiting the same range of sizes as observed in the absence of PAM.
In order to better understand the nature of the interaction between AgNPs and the organic matrix, an FT-IR study was performed (Fig. S5†). Both TMPLig/PAM and AgNP@TMPLig/PAM spectra were similar, and essentially composed of characteristic lignin peaks.51 The presence of silver did not significantly affect the organic matrix. The oxidation state of silver in the samples supported on PAM was investigated using XPS. All samples, irrespective of the lignin source, indicated the presence of Ag(0) with 3d5/2 peaks above 368 eV: at 368.5 eV for AgNP@KLig/PAM and at 368.1 eV for AgNP@TMPLig/PAM (Fig. S6 and S7†).52 The PXRD patterns of AgNP@KLig/PAM and AgNP@TMPLig/PAM showed a small number of peaks, which could all be attributed to crystalline metallic silver (Fig. S8 and S9†). The low signal to noise ratio was due to the small size of the silver crystallites, in accordance with TEM results. Specifically, the PXRD pattern of AgNP@KLig/PAM showed four clear peaks for Ag(111), Ag(200), Ag(220), and Ag(311) X-ray reflections. For the AgNP@TMPLig/PAM composite only the (111) and (220) X-ray reflections of silver were visible.53 Such results are in agreement with the XPS analysis and clearly confirm the presence of Ag(0) in all samples, validating the expected role of lignin as a solid reducing agent for Ag(I).
As both sources of lignin were equivalent and kraft lignin is readily accessible on a multi-ton scale, AgNPs@KLig/PAM was tested for ability to act as an antibacterial water filter (Scheme 2). A porous plug was prepared by placing 50 mg of AgNP@KLig/PAM into a plastic 5 mL syringe with a syringe filter with a 5.0 μm pore size, i.e. sufficiently large to allow bacteria to pass through it easily. The plug was then washed by passing through it 20 mL of water, at a slow flow. A 2 mL sample of a bacterial dispersion containing 1 × 108 cfu was then quickly passed through the plug using the syringe plunger. As a control, the same solutions were passed under exactly the same conditions through syringe filters containing 50 mg of KLig/PAM, the composite polymer mixture not containing any silver. The collected filtrates were used to make serial dilutions which were then plated on the appropriate agar plates. The plates were incubated overnight at 37 °C and compared against control ones (Fig. 2 and S10–S13†). For this work, we tested 5 distinct bacteria, namely Enterococcus faecium ATCC 19434, Staphylococcus aureus ATCC 43300, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 700721, and Escherichia coli ATCC 25922. In all cases, no bacterial colonies were observed on agar plates incubated with solutions that were passed though the silver-containing composites. In contrast, normal colony growth was observed for agar plates that were incubated with the solutions passed through the metal free KLig/PAM mixture. Overall, the AgNP@KLig/PAM composite proved highly efficient as an antibacterial agent, killing 99.99% of the bacteria. The results were equally good for all herein explored bacterial strains, including the multi-drug resistant S. aureus ATCC 43300 and K. pneumoniae ATCC 70721. The investigated bacterial included both Gram negative (P. aeruginosa, K. pneumoniae, E. coli) and Gram positive (S. aureus, E. faecium) bacteria. A blank measurement was also performed by passing the solution through the syringe in absence of any polymer. After incubating agar plates with that solution, normal bacterial activity was again observed. The described results prove that the observed antibacterial activity originates directly from the presence of silver contained within the composite: while the presence of lignin is essential as a reducing environment for producing AgNPs, it generates no antibacterial activity on its own, under the conditions we used. In order to establish the potential recyclability of this material, we ran this test 5 times in a row on the same plug and noticed that, each time, the bacterial activity was completely eradicated.
 | ||
| Scheme 2 Illustration of the antimicrobial tests performed on AgNP@KLig/PAM against the KLig/PAM control. | ||
 | ||
| Fig. 2 Photo of agar plates incubated with a solution of K. pneumonia after being passed through an AgNP@KLig/PAM equipped filter plug (top), and a KLig/PAM equipped filter plug (bottom). | ||
For each sample, the amount of active Ag(I) leached in solution was evaluated by analyzing a digested sample of the filtrate by ICP-OES. A low value of 0.31 ppm of Ag was measured, which is close to the lowest concentrations known to still ensure efficient antibacterial activity.14
All the described results are consistent with the following scheme: lignin, a material produced in large scale from biomass and which is largely underutilized can be used as an efficient reducing agent for silver salts, replacing more aggressive and sensitive reagents, such as NaBH4. While the use of lignin for AgNP production has been reported in solution-based systems, they suffer from limitations including the use of very basic conditions, the poor solubility of lignin and the necessity to use a solvent.29,30 The present study shows how solvent free method can allow access to materials of high added value directly by mechanochemistry. The resulting material have activity similar to previously reported antibacterial silver nanomaterials, while maintaining a low level of leaching.15–18 This result is consistent with our previous study on other noble metals, including Au.37 The accessibility of kraft lignin makes it particularly suitable for this application. As it is known that the mechanism of antibacterial activity of AgNPs relies on the local formation of Ag(I) ions by oxidation under aerobic conditions,14 the herein presented results indicate that mechanochemically prepared AgNPs@KLig/PAM is capable of generating sufficient amounts of Ag(I) to eradicate all tested bacterial types, at a high flow rate (of the order of 1 mL s−1), while maintaining a low concentration of Ag(I) in the effluent. This approach to water disinfection is particularly appealing because of the low resistance of bacteria towards silver, whose persistent and long-known bactericidal activity is explained by the fact that Ag+ ions act on a broad range of targets, making it difficult for microorganisms to develop immunity.54
Conclusions
In conclusion, we have developed a fast, inexpensive, and a highly atom-economical method for producing antibacterial filters. We established that solvent-free milling of Ag(I) salts with a mixture of lignin as a reducing agent and PAM as a support polymer afforded AgNPs nicely embedded into an organic matrix. Both kraft and thermomechanically produced lignin proved suitable for this purpose. The presented method affords several key advantages in the prospect of promoting sustainable synthesis of functional nanomaterials: (1) a lower energy input compared to conventionally used techniques, as a typical laboratory mill operates with the energy input comparable to a domestic light bulb (∼50 W per sample);44 (2) the solid material is produced pure and without any solvent use, circumventing the need for energy-intensive evaporation and drying steps, (3) lignin is the key component in this synthesis and is readily available in large quantities in most regions of the world. For all these reasons, this method ranks very high based on all eight pointers highlighted by Varma, Kirwan and co-workers for the sustainability evaluation of AgNPs synthetic methods.24 This method opens an affordable and sustainable avenue for the generation of point-of-use filters using local resources.Acknowledgements
We thank the Natural Science and Engineering Research Council of Canada (NSERC) Discovery Grant program, the Canada Foundation for Innovation (CFI), the Canada Research Chairs (CRC), the Fonds de Recherche du Québec – Nature et Technologies (FRQNT) Equipe program, the Centre for Green Chemistry and Catalysis (CGCC), NSERC-Collaborative Research and Training Experience (CREATE) in Green Chemistry and McGill University for their financial support. Md Nur Alam and Theo G. M. van de Ven are thanked for providing samples of TMP lignin. Kenward Vong and Karine Auclair are acknowledged for providing all bacteria lines, microbiology assistance and training.Notes and references
- W. H. Organization, Mortality and burden of disease from water and sanitation, http://www.who.int/gho/phe/water_sanitation/burden/en/index.html, accessed July 8, 2014.
- J. W. Alexander, Surg. Infect., 2009, 10, 289–292 CrossRef PubMed.
- M. Rai, S. Deshmukh, A. Ingle and A. Gade, J. Appl. Microbiol., 2012, 112, 841–852 CrossRef CAS PubMed.
- H. C. Neu, Science, 1992, 257, 1064–1073 CAS.
- C. Marambio-Jones and E. M. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- A. Panáček, L. Kvitek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. J. Nevečná and R. Zboril, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef PubMed.
- C. Baker, A. Pradhan, L. Pakstis, D. J. Pochan and S. I. Shah, J. Nanosci. Nanotechnol., 2005, 5, 244–249 CrossRef CAS PubMed.
- H. H. Lara, N. V. Ayala-Núnez, L. D. C. I. Turrent and C. R. Padilla, World J. Microbiol. Biotechnol., 2010, 26, 615–621 CrossRef CAS.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- F. Solsona and J. P. Méndez, Water disinfection, Pan American Center for Sanitary Engineering and Environmental Sciences, Pan American Health Organization, Regional Office of the World Health Organization, 2003 Search PubMed.
- S. Corbett, in New York Times, Arthur Ochs Sulzberger, New York City, 2008, December 24 Search PubMed.
- J. Arquiette, M. P. Stevens, J. M. Rabb, K. Sanogo, P. Mason and G. M. L. Bearman, Advances in Public Health, 2014, 2014, 734254 CrossRef.
- J. S. Kim, E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D. H. Jeong and M.-H. Cho, Nanomedicine: Nanotechnology, Biology and Medicine, 2007, 3, 95–101 CrossRef CAS PubMed.
- Z.-M. Xiu, Q.-B. Zhang, H. L. Puppala, V. L. Colvin and P. J. J. Alvarez, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS PubMed.
- T. A. Dankovich and D. G. Gray, Environ. Sci. Technol., 2011, 45, 1992–1998 CrossRef CAS PubMed.
- I. Yakub and W. O. Soboyejo, J. Appl. Phys., 2012, 111, 124324 CrossRef.
- P. Jain and T. Pradeep, Biotechnol. Bioeng., 2005, 90, 59–63 CrossRef CAS PubMed.
- I. Sheet, H. Holail, Z. Olama, A. Kabbani and M. Hines, Int. J. Curr. Microbiol. Appl. Sci., 2013, 2, 1–11 Search PubMed.
- V. A. Oyanedel-Craver and J. A. Smith, Environ. Sci. Technol., 2007, 42, 927–933 CrossRef.
- E. N. Kallman, V. A. Oyanedel-Craver and J. A. Smith, J. Environ. Eng., 2010, 137, 407–415 CrossRef.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed.
- C. J. Murphy, J. Mater. Chem., 2008, 18, 2173–2176 RSC.
- M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS.
- M. Cinelli, S. R. Coles, M. N. Nadagouda, J. Błaszczyński, R. Słowiński, R. S. Varma and K. Kirwan, Green Chem., 2015, 17, 2825–2839 RSC.
- V. K. Sharma, R. A. Yngard and Y. Lin, Adv. Colloid Interface Sci., 2009, 145, 83–96 CrossRef CAS PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS PubMed.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2008, 10, 859–862 RSC.
- M. C. Moulton, L. K. Braydich-Stolle, M. N. Nadagouda, S. Kunzelman, S. M. Hussain and R. S. Varma, Nanoscale, 2010, 2, 763–770 RSC.
- S. Hu and Y.-L. Hsieh, Carbohydr. Polym., 2015, 131, 134–141 CrossRef CAS PubMed.
- S. Hu and Y.-L. Hsieh, Int. J. Biol. Macromol., 2016, 82, 856–862 CrossRef CAS PubMed.
- A. Li, M. Kaushik, C.-J. Li and A. Moores, ACS Sustainable Chem. Eng., 2016, 4(3), 965–973 CrossRef CAS.
- B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259–281 CrossRef CAS.
- B. M. Trost, Acc. Chem. Res., 2002, 35, 695–705 CrossRef CAS PubMed.
- A. Kumar, P. K. Vemula, P. M. Ajayan and G. John, Nat. Mater., 2008, 7, 236–241 CrossRef CAS PubMed.
- M. Kaushik, A. Y. Li, R. Hudson, M. Masnadi, C.-J. Li and A. Moores, Green Chem., 2016, 18, 129–133 RSC.
- A. Baksi, M. Gandi, S. Chaudhari, S. Bag, S. S. Gupta and T. Pradeep, Angew. Chem., Int. Ed., 2016 DOI:10.1002/ange.201510122.
- M. J. Rak, T. Friscic and A. H. Moores, Faraday Discuss., 2014, 170, 155–167 RSC.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- D. Stewart, Ind. Crops Prod., 2008, 27, 202–207 CrossRef CAS.
- J. H. Lora and W. G. Glasser, J. Polym. Environ., 2002, 10, 39–48 CrossRef CAS.
- F. J. Lu, L. H. Chu and R. J. Gau, Nutr. Cancer, 1998, 30, 31–38 CrossRef CAS PubMed.
- P. Balaz, M. Achimovicova, M. Balaz, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutkova, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- D. Debnath, S. H. Kim and K. E. Geckeler, J. Mater. Chem., 2009, 19, 8810–8816 RSC.
- M. J. Rak, N. K. Saade, T. Friscic and A. Moores, Green Chem., 2014, 16, 86–89 RSC.
- T. Kleine, J. Buendia and C. Bolm, Green Chem., 2013, 15, 160–166 RSC.
- A. Fujimoto, Y. Matsumoto, H.-M. Chang and G. Meshitsuka, J. Wood Sci., 2005, 51, 89–91 CrossRef CAS.
- Z.-H. Wu, M. Sumimoto and H. Tanaka, Holzforschung, 1994, 48, 395–399 CrossRef CAS.
- F. S. Chakar and A. J. Ragauskas, Ind. Crops Prod., 2004, 20, 131–141 CrossRef CAS.
- L. C. Vander Wielen, Ph.D. thesis, Georgia Institute of Technology, 2004.
- F. A. Andersen, Internet J. Toxicol., 2005, 24, 21–50 CrossRef CAS PubMed.
- O. Derkacheva and D. Sukhov, Macromol. Symp., 2008, 265, 61–68 CrossRef CAS.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X Ray Photoelectron Spectroscopy, Physical Electronics, Eden Prairies, Minnesota, 1995 Search PubMed.
- S. Basavaraja, S. Balaji, A. Lagashetty, A. Rajasab and A. Venkataraman, Mater. Res. Bull., 2008, 43, 1164–1170 CrossRef CAS.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data, bacterial plates, TEM pictures, PXRD, FTIR, and XPS. See DOI: 10.1039/c6ra03711a |
| This journal is © The Royal Society of Chemistry 2016 |