Glycine modified graphene oxide as a novel sorbent for preconcentration of chromium, copper, and zinc ions from water samples prior to energy dispersive X-ray fluorescence spectrometric determination
Abstract
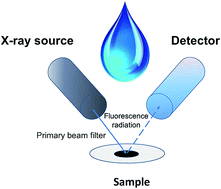
A novel and selective sorbent for micro-solid phase extraction was synthesized by chemical functionalization of graphene oxide with glycine. The structure of this nanomaterial, referred to as GO-Gly, was confirmed by Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, and scanning electron microscopy. GO-Gly was used for preconcentration of chromium, zinc, and copper ions from water samples prior to their determination by energy dispersive X-ray fluorescence spectrometry (EDXRF). The proposed procedure is based on dispersion of micro amounts of GO-Gly in aqueous samples. After adsorption of metal ions on its surface, samples were filtered under vacuum and then membrane filters were directly submitted to energy dispersive X-ray fluorescence spectrometric measurements. In order to obtain optimal preconcentration conditions, some parameters affecting sorption process, such as pH, amount of GO-Gly, sorption times, and sample volume, were examined. Under optimal conditions the calibration curves were linear in a 1–150 ng mL−1 range with recoveries higher than 97%. The obtained detection limits for Cr(III), Zn(II), and Cu(II) determinations are 0.15, 0.07, and 0.08 ng mL−1, respectively. A relative standard deviation of the proposed procedure (at a 10 ng mL−1 level for n = 10) is lower than 2.3%. The proposed method was successfully applied for determination of Cr(III), Zn(II), and Cu(II) ions in water samples.

 Please wait while we load your content...
Please wait while we load your content...