Pervaporation dehydration of an acetone/water mixture by hybrid membranes incorporated with sulfonated carbon molecular sieves†
Abstract
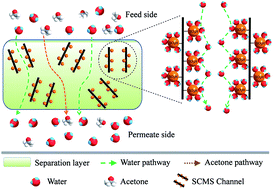
In this study, hybrid membranes incorporated with sulfonated carbon molecular sieves were fabricated and utilized for pervaporation dehydration of an acetone/water mixture. First, carbon molecular sieves (CMS) were synthesized via a hard template method, and sulfonated by concentrated H2SO4 to prepare sulfonated carbon molecular sieves (SCMS). The pore structure, surface properties and morphological features of CMS and SCMS were examined by XRD, FT-IR, TEM, EDX, nitrogen adsorption–desorption and HRTEM. The results showed that the obtained CMS had an ordered mesoporous structure with cylindrical channels and large surface area. After sulfonation, the surface properties of CMS changed from hydrophobic to hydrophilic. Then, SCMS with different contents were incorporated into a chitosan (CS) matrix to fabricate CS-SCMS hybrid membranes. The incorporation of SCMS can simultaneously create more water transportation pathways due to the ordered mesoporous structure and increase the number of hydrophilic sites due to the sulfonic acid groups within the membranes. When the content of SCMS was 2 wt%, an optimum separation performance with a separation factor of 832 and a permeation flux of 1.81 kg m−2 h−1 was acquired, which was increased by 2 times for the separation factor and 1.67 times for the permeation flux compared with those of the pure CS membrane. Meanwhile, the hybrid membrane showed good long-term stability.

 Please wait while we load your content...
Please wait while we load your content...