Removal of anionic azo dye from water with activated graphene oxide: kinetic, equilibrium and thermodynamic modeling
Yongfu Guo*,
Juan Deng,
Junyan Zhu,
Chao Zhou,
Caiyun Zhou,
Xiaoji Zhou and
Renbi Bai
Center for Separation and Purification Materials & Technologies, Suzhou University of Science and Technology, Suzhou 215009, P. R. China. E-mail: yongfuguo@mail.usts.edu.cn; Tel: +86-512-68092987
First published on 14th April 2016
Abstract
In order to improve the specific surface area value and adsorption capacity of graphene oxide (GO), activated GO (GOKOH) was successfully prepared by high temperature solid state activation with potassium hydroxide, and was used as the adsorbent for the removal of the anionic dye orange IV from water. The obtained adsorbent was characterized by XRD, FTIR, zeta potential, BET and SEM, respectively. The results show that GOKOH had an extraordinary specific surface area of 672.5 m2 g−1 and large adsorption capacity of 606.1 mg g−1 for anionic orange IV, which were much higher than those of GO synthesized by the Hummers method. The activation method can remarkably reduce the amount of electronegative charges on the surface of GO and promote the interaction between GO molecules and anionic dye molecules. The adsorption process was endothermic and spontaneous in nature, following the pseudo-second-order kinetics and the Langmuir isotherm models. The present adsorption studies of anionic dyes revealed the potential of GOKOH to be utilized as adsorbents with super adsorption capacity for water purification.
1. Introduction
Nowadays, the contamination of water resources by a variety of pollutants from industrial production has been a global issue.1 Thousands of tons of pollutants and sewage are poured into natural rivers and lakes every day. Organic dyes with carcinogenic and mutagenic effects are one of the main water pollutants which can cause serious damage to human beings and aquatic biota.2 It was reported that the amount of total dyes exceeds 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year.3 One of the major problems is that the increase of water color can impede the penetration and transmission of incident light, which affects photosynthesis and harms aquatic ecosystems.
000 tons per year.3 One of the major problems is that the increase of water color can impede the penetration and transmission of incident light, which affects photosynthesis and harms aquatic ecosystems.
During the past decade, the development of advanced adsorbent materials in the field of environmental remediation has obtained much attention. Nowadays, numerous adsorbents, such as activated carbon,4,5 graphene oxide,2,6 graphite oxide,7 carbon nanotubes,8 iron oxides,9 molecular sieves,10 polymer materials,11 chitosan,3,12 guar gum13 and aerogels14 have been developed and frequently used to treat dye pollutants. Among these adsorbents, graphene oxide (GO), a kind of highly oxidized and chemically modified graphene material, has attracted considerable interesting due to its superior properties, such as large theoretical specific surface area of 2630 m2 g−1,3 strong mechanical strength, good water solubility and changeable chemical activity.
GO is a layered and oxygenated graphene sheet with oxygen-containing functional groups, such as –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –COOH on the surface of GO nanosheets. These functional groups are essential for the high-adsorption of pollutants.15 Owing to its unique structure and excellent properties, GO offers great potential to be used as an excellent adsorbent for nature water remediation.
O, and –COOH on the surface of GO nanosheets. These functional groups are essential for the high-adsorption of pollutants.15 Owing to its unique structure and excellent properties, GO offers great potential to be used as an excellent adsorbent for nature water remediation.
However, GO normally prepared by modified Hummers method or other methods exhibits lower specific surface area values compared with the theoretical values predicted, which results in the reduction of its adsorption capacity for the removal of pollutants and limitation of its application as adsorbents.1,7,16
In order to improve this disadvantage, a facile method for increasing the specific surface area and adsorption capacity of GO is High Temperature Solid State Activation method (HTSSA). It was reported that this method with alkali metal hydroxides as the activating agent can remarkably increase the specific surface areas and porosities of some carbon materials, such as lignins,17 carbon nanotubes (CNTs),18 carbon nanofibers (CNFs),19,20 graphene nanosheets21,22 and graphite nano fibers (GNFs).23,24
Among the alkali metal hydroxides, potassium hydroxide (KOH) is one of the most effective activating agents in producing activated carbon materials. These activated materials are mainly employed in the electrochemical fields, particularly for fabrication of electrodes for fuel cell applications. Few studies on the adsorption of pollutants with these activated materials as adsorbents have been reported.
Consequently, this work employed GO and KOH as the basic material and activating agent, respectively. The objectives of this study were to: (i) prepare activated adsorbents with higher specific surface area value and apply them as adsorbents to remove anionic dye orange IV from aqueous solution; (ii) explore the effects of adsorbent dosage, solution pH, reaction temperature and reaction time on orange IV; and (iii) discuss the kinetics, equilibrium modeling and thermodynamics of adsorption for orange IV. This study provides a novel and promising method for the application of GO in environmental remediation.
2. Materials and experiment methods
2.1. Chemicals and materials
Graphite oxide (manufactured by standard Hummers method) was purchased from Nanjing XFNANO Materials Tech Co., Ltd. Orange IV was obtained from Sinopharm Chemical Reagent Co., Ltd. KOH, sodium hydroxide (NaOH) and hydrochloric acid (HCl) were purchased from Chinasun Specialty Products Co., Ltd. All chemicals were analytical grade products without further purification. The structures and characteristics of orange IV are displayed in Table 1.2.2. Preparation of activated GO
Initially, a suspension of GO (1 g graphite oxide powder dissolved in 1000 mL deionized water) was obtained after ultrasonic treatment for 13 h. Subsequently, the suspension was dried for 24 h in a freeze drier (SP Scientific, USA) after heating concentration. Then as-obtained sample was non-activated GO powder and was reserved in a sealed bag.The activated GO was prepared as followings: a certain amount of the mixtures of non-activated power GO and solid KOH was placed on a Ni-pan in a horizontal tubular furnace. The ratio of GO and KOH was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (g g−1). The activation conditions were 800 °C for 1 h with the heating rate of 5 °C min−1 under nitrogen flow of 75 mL min−1. The activated product was washed with distilled water until the solution was neutralized and then dried at 105 °C. The as-prepared sample was designated as GOKOH.
4 (g g−1). The activation conditions were 800 °C for 1 h with the heating rate of 5 °C min−1 under nitrogen flow of 75 mL min−1. The activated product was washed with distilled water until the solution was neutralized and then dried at 105 °C. The as-prepared sample was designated as GOKOH.
2.3. Sample characterizations
The X-ray diffraction (XRD) patterns of prepared GO and GOKOH particles were recorded on benchtop XRD system (D\max-2550, Japan) with Cu Kα radiation. The diffracted intensities were recorded from 5° to 50° at 2θ angles. A scanning electron microscope (SEM, PhenomG3, Phenom-world, USA) was used to observe the surface morphologies of the materials studied at an accelerating voltage of 20 kV. BET (Brunauer–Emmett–Teller) specific surface area along with total pore volume and average pore diameter of the materials were determined using a nitrogen adsorption apparatus (ASAP2020, Quantachrome, USA). For the FTIR spectroscopic measurements, GO and GOKOH powder were mixed with spectroscopic grade potassium bromide and spectra were recorded in the range of 500–4000 wave number (cm−1) on FTIR Spectrum (Nicolet-6700, Thermo, USA).In order to study the adsorption mechanism under the influence of pH, the zeta potential values of each absorbent in various pH solutions were obtained by electrophoresis method (ZetaPALS, Brookhaven, USA). The concentrations of orange IV before and after adsorbed by GO and GOKOH were determined according to the absorbance at the wavelength of 476 nm by a UV-vis spectrometer (UV3600, Shimadzu, Japan). Raman spectra were recorded on a microscope Raman spectrometer (DRX, Thermo Fisher Scientific, USA) with a 532 nm laser. The intensity of a Raman peak was extracted from the maximum value after baseline subtraction over corresponding spectral range.
2.4. Batch adsorption experiments
The adsorption and kinetic properties of orange IV removal by GO and GOKOH were determined in batch experiments. In the adsorption experiments, a certain amounts of GO and GOKOH were added into 100 mL separate flasks filled with 20 mL raw solution of orange IV with different concentrations. The mixture was then shaken for 24 h at room temperature using a shaker water bath at a constant rate of 200 rpm. After that, the solution was filtrated through 0.45 μm filter to obtain supernatant liquid. The obtained supernatant was collected for subsequent analysis to determine the residual dye concentration in the adsorbed solution by using an UV-vis spectrometer. Blank solutions (without any adsorbents) were treated similarly.The adsorption capacities and removal efficiencies of orange IV onto GO and GOKOH were calculated using the following equations:
 | (1) |
In the adsorption kinetic experiments, the adsorption capacity can be calculated using the following equation:
 | (2) |
Removal efficiency E can be calculated by eqn (3):
 | (3) |
Before analyzing the dye concentrations, a calibration curve for orange IV was depicted to ensure precision of measurements. All the experiments were performed in triplicate and the average of the data was taken for final value and later calculations.
2.5. Recycling test
For the desorption and regeneration study, 3 mg of GO and GOKOH was added into separate 20 mL of 100 mg L−1 orange IV solution and then shaken for 8 h at room temperature and pH of 7 using a shaker water bath at a constant rate of 200 rpm. After adsorption, the adsorbents were collected by filtration with 0.45 μm filter. Then the adsorbents were dispersed into 20 mL of ethanol solution (10% vol. HCl acid), and stirred for 30 min after sonicated for 15 min. After treated with ethanol solution, the adsorbents were washed with deionized water for 3 times. The regenerated GO and GOKOH were then used for repeated adsorption/desorption cycles for five times to study their recyclability.3. Results and discussion
3.1. Characterization of GO and GOKOH
The XRD spectrum provides a conclusive proof for the degree of activation as the layer distances of non-activated GO and the activated GOKOH are obviously different, as shown in Fig. 1.The diffraction peaks of GOKOH were found at approximately 2θ = 12.3, 22.6 and 44.6 which corresponded to the layer-to-layer distance (d-spacing) of 0.73 nm (d001), 0.33 nm (d002, not obvious) and 0.21 nm (d100). The d-spacing was significantly reduced from 0.90 nm (d001) after GO was activated, which indicated that some oxygen-containing functional groups were removed from the GO sheets. Owing to the negative surface of GO resulted from these functional groups with negative charge, the decrease of the amount of these polar functional groups contributed to the reduction of surface electrical negativity of GO, which was advantageous to the adsorption of anionic dyes.
In addition, the crystalline size of the GOKOH was smaller than that of the non-activated GO as shown by the larger X-ray peak half-width of the GOKOH. It indicates that the activated GO had smaller crystal grain and more structure defects,18,25 which could give the activated GO a higher specific surface area that was favorable for the adsorption of orange IV on the surface of GOKOH.
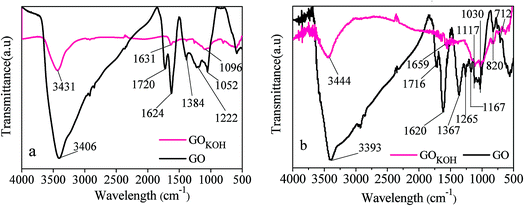
In order to further explore the surface characteristics of GOKOH sheets, FTIR analysis was performed and the revealed spectrum can be seen in Fig. 2. The bands at near 3400 cm−1 are attributed to the –OH stretching vibrations arising from hydroxyl groups in GO and GOKOH sheets.28 The peaks at about 1700 cm−1 can be assigned to the skeletal vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carbonyl and carboxyl groups.26,27 The peaks from 1620 to 1660 cm−1 are associated with the stretching vibration of C
O in the carbonyl and carboxyl groups.26,27 The peaks from 1620 to 1660 cm−1 are associated with the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.28 The adsorption bands at near 1380 cm−1 are the blending vibration of C–O in the carboxyl.1
C.28 The adsorption bands at near 1380 cm−1 are the blending vibration of C–O in the carboxyl.1
The peaks at 1265 and 1222 cm−1 can be ascribed to the vibration of ionic sulphate and C–O in the epoxide group, respectively.3,27 The peak at 1167 cm−1 and the peaks from 1030 to 1117 cm−1 in GOKOH are all associated with the stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in orange IV adsorpted.3 The two characteristic peaks at 1052 and 1095 cm−1 are assigned to the vibration of C–O in the alkoxy group.27 The characteristic peaks at 1052 and 1096 cm−1 are assigned to the vibration of C–O in the epoxy or alkoxy groups.1,27 In particular, the strong characteristic absorptions from 712 to 820 cm−1 in GO (two peaks) and GOKOH (three peaks), due to the sulfo groups, were assigned C–O–S bond stretching.28,29
O in orange IV adsorpted.3 The two characteristic peaks at 1052 and 1095 cm−1 are assigned to the vibration of C–O in the alkoxy group.27 The characteristic peaks at 1052 and 1096 cm−1 are assigned to the vibration of C–O in the epoxy or alkoxy groups.1,27 In particular, the strong characteristic absorptions from 712 to 820 cm−1 in GO (two peaks) and GOKOH (three peaks), due to the sulfo groups, were assigned C–O–S bond stretching.28,29
The obvious change was that the sharp decline of the amount of hydroxyl groups in GOKOH. The results shown in Fig. 2 indicate that the amount of oxygen-containing functional groups, especially hydroxyl groups, were significantly reduced after GO activated by HTSSA method, which was also greatly beneficial to the reduction of surface electrical negativity of GO. The results were consistent with the XRD data.
Compared with the FTIR characterizations before adsorption, the difference of FTIR data of GO and GOKOH after adsorption was not notable, indicating the adsorption effect on the structures of GO and GOKOH relatively small. However, it should be noted that the peak intensities from 600 to 2000 cm−1 were strengthen after GO activated, and some new peaks (1030, 1117 and 1167 cm−1) were observed, indicating that orange IV were absorbed onto GO and GOKOH. Moreover, the presence of C–O–S bond suggested some chemical reactions occurred between orange IV and adsorbents.
Zeta potential is one of the parameters that connect to the exterior charges of the absorbents. Fig. 3 elucidated the dissociation processes of functional groups on the surface of absorbents under the influence of H+/OH− ionic strength. All the zeta potentials of GO and GOKOH were negative over the experimental pH range. The tendency of the exterior charge of GOKOH was assimilate to that of GO in the experimental pH regions. Namely, the exterior zeta potentials on the surface of the two samples decreased with the increasing of pH values.
The anionic dyes adsorption may vary at various pH values since the pH affects the structures and charges of both adsorbent and adsorbate surfaces. When pH value increased, the negative charges of the two adsorbents increased too. These charges offered electrostatic repulsion which was unfavorable for anionic dyes reacting with functional groups.
The exterior potential values of GO were lower than that of GOKOH at the same point of pH. For example, the potential value decreased by 39.8% at pH = 7 after GO was activated, which meant more number of acidic groups with negative charges were loaded on the exterior surface of GO than that on the exterior surface of GOKOH. In other words, the amount of oxygen-containing functional groups on the surface of GOKOH decreased after GO was activated with KOH, which offered a more possible affinity for GOKOH to orange IV compared to GO. This result validated the previous analysis.
The Raman spectra of two samples were characterized by two main features of G and D modes, as shown in Fig. 4. The Raman spectra of GOKOH showed a G-band at ∼1566 cm−1 and a D-band at ∼1350 cm−1. The G-band was associated with the vibration of sp2 carbon atoms, and the D-band originated from the vibration of sp3 defective or out-of-order graphitic carbon atoms.
The intensity ratio of D to G bands (ID/IG) is an index for evaluating the defects of graphene materials. The ID/IG of GOKOH (ID/IG = 0.92) was larger than that of GO (ID/IG = 0.83), reflecting a certain defect density of GOKOH and also high activation degree of GO by KOH.21,30 This result was also confirmed by XRD data.
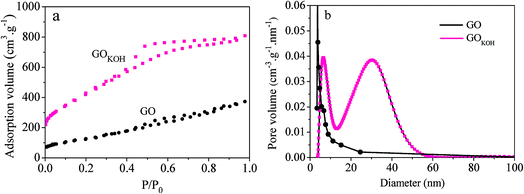
Fig. 5a is the nitrogen adsorption isotherms of GO and GOKOH. The isotherms of GOKOH activated with KOH exhibited obvious H4 hysteresis loops, suggesting mesoporous features.31 The pore size distributions (shown in Fig. 5b) calculated by BJH method showed main pore sizes around 5–50 nm for GOKOH.
According to the N2 adsorption and desorption analysis, the BET surface area and BJH desorption cumulative pore volume of GOKOH were 672.5 m2 g−1 and 0.668 cm3 g−1, respectively, much higher than those of GO of 35.5 m2 g−1 and 0.022 cm3 g−1.
The BET results show that the method of HTSSA method can significantly increase the BET specific surface area and pore volume of GO. And this method may contribute to the adsorption for anionic dyes onto GOKOH.
The micrographs in Fig. 6 depicted the surface morphologies of two absorbent materials. The surface of GO (Fig. 6a) exhibited many wrinkles and a more silk-like wave sheet structure which was the unique characteristic of graphene. However, the surface morphology of GOKOH (Fig. 6b) appeared many sheet structures with a great number of widened pores with some cracks corroded by KOH. These sheet structures and widened pores were favorable for the increase of specific surface area of GO.
The EDX of the two materials was also used to qualitative analyse the contents of each element in GO and GOKOH, shown in Fig. 6c and d. According to the EDX data, it can be seen that the oxygen content obviously reduced from 32.5 wt% to 5.2 wt% after GO activated. The results indicated that the content of oxygen-containing functional groups on the surface of GO reduced sharply. Simultaneously, some potassium ions had been doped into/onto GOKOH.
3.2. Adsorption performance test
Fig. 7 shows the adsorption capacities of GO and GOKOH in the pH range of 3–11. The results show that the adsorption capacity for orange IV gradually decreased with the increase of pH. However, the difference of adsorption capacities of GO or GOKOH was not notable at pH from 3 to 11.
 | ||
| Fig. 7 Adsorption capacities under various pH conditions. Condition: C0 = 100 mg L−1, V = 20 mL, T = 298 K, W = 3 mg and contact time t = 24 h. | ||
The reason was follows: as the pH increased, more electronegative charges were formed on the surface of adsorbents under alkaline conditions (see the zeta potential shown in Fig. 3).25,32 As an anionic azo dye with sulfonic acid group, orange IV has a pka of 2.0.33 And the pka of the sodium sulfonate groups (–SO3Na, i.e. sulfonic groups) present in the azo dye is below pH 1.0. Orange IV dissociated to the sodium ion and sulfonate anion and was already negatively charged at pH window tested, resulting in the occurrence of repulsive interaction between adsorbents and orange IV.34,35 Higher the pH of the solution was, higher the repulsion between adsorbents and orange IV was and lower the adsorption capacity for dyes was. However, it should be noted that sulfonate anions (R–SO3) can react with protons to the neutral form (R–SO3H) at lower pH, which is unfavorable for the adsorption of anionic dyes under low pH. Thus, the change of adsorption capacities for orange IV with adsorbents of GO or GOKOH was not notable at pH window tested.
However, the adsorption capacity of GOKOH was more than 4 times as much as that of GO under the same condition of pH. It indicates that the reduction of electronegative charges on the surface of GOKOH was favorable for the adsorption of anionic dye orange IV.
In general, the pH-behavior observed was the expectable based on considerations of zeta potential, but the effect of pH on the adsorption of orange IV was not remarkable. Taking the adsorption capacity and practical application possibility into consideration, the solution pH of 7 was chosen as one of the factors of the subsequent experiments.
 | ||
| Fig. 8 Effects of adsorbent dosage on the adsorption capacity and removal efficiency. Condition: C0 = 100 mg L−1, V = 20 mL, T = 298 K, pH = 7 and contact time t = 24 h. | ||
Fig. 8 exhibited the strong correlation among the adsorption capacity, removal efficiency and the ratio of solid to liquid. The results reveal that the removal efficiency increased rapidly with an increasing ratio of solid to liquid, which was mainly due to the active sites enhanced with an increasing amount of GO and GOKOH.
By comparison, the changes of removal efficiency with GOKOH as adsorbent were faster than those with GO as adsorbent. The removal efficiency of orange IV reached 94.6% with GOKOH as absorbent and this value was much higher than that of 32.4% with GO as adsorbent under the condition of the solid to liquid ratio of 0.3. The result validates again that HTSSA method with KOH can efficiently improve the adsorption capacity of GO for anionic dye.
However, the adsorption capacity slowly reduced as increasing adsorbent dose. This was due to too more active sites exceeding the demand of the saturated adsorption, which made a large number of effective active sites not used, resulting in the reduction of adsorption capacity.
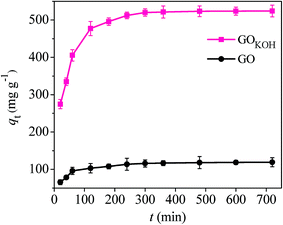 | ||
| Fig. 9 Adsorption capacity of orange IV as a function of adsorption time t. Condition: C0 = 100 mg L−1, V = 20 mL, T = 298 K, W = 3 mg and pH = 7. | ||
The instantaneous dye adsorption (0–1 h) onto GO and GOKOH, governed by fast external diffusion and mainly surface adsorption,7 was followed by a milder and gradual ascend (1–3 h), resulting in an equilibrium state plateau from 3 to 24 h.
The increase in adsorption capacity with increasing contact time was attributed to more available time for pollutants to interact with adsorption sites on the surface or inside of GO and GOKOH.
3.3. Adsorption kinetics
Adsorption kinetic studies are essential for the further perspective for the adsorption mechanisms of absorbents to be applied on the adsorption rate and capacity enhancement. Hence, the pseudo-first-order equation, pseudo-second-order equation, Elovich and intra-particle diffusion equations were applied to describe the kinetics of orange IV adsorption onto GO and GOKOH.The adsorption kinetic studies were carried out with orange IV concentration of 100 mg L−1, ratio of solid to liquid of 0.15 g L−1, agitation rate of 200 rpm, pH of 7 and absolute temperature of 298 K. Residual dye concentration was measured at 20, 40, 60, 120, 180, 240, 300, 360, 480, 600 and 720 minutes, separately.
The fitting of pseudo-first-order can be utilized by eqn (4):
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (4) |
 | (5) |
Compared with former adsorption kinetic models, the assumption of heterogeneously distributed adsorption energy on the surface of absorbents makes Elovich equation more widespread in indicating the essence of adsorption mechanism, and Elovich data will not be more easily influenced by measurement errors. When considered instantaneous adsorption, the model can be described by eqn (6):
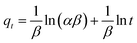 | (6) |
When considered solute diffusion in the intercrystalline of adsorbents, especially for porous materials like carbon materials, the intra-particle diffusion model usually is used. A simplified equation is shown below:
| qt = kdit0.5 + Ci | (7) |
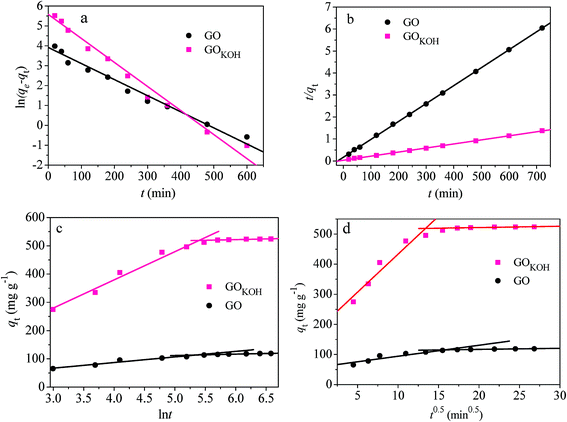 | ||
| Fig. 11 Pseudo-first-order kinetics (a), pseudo-second-order kinetics (b), Elovich kinetics (c), and intra-particle diffusion kinetics (d) for adsorption of orange IV onto GO and GOKOH. | ||
| Adsorbent | Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| qe,exp (mg g−1) | qe,cal (mg g−1) | k1 (L min−1) | R2 | qe,cal (mg g−1) | k2 (L min−1) | R2 | |
| GO | 119.1 | 50.4 | 0.008 | 0.977 | 122.4 | 0.014 | 0.999 |
| GOKOH | 524.1 | 265.8 | 0.012 | 0.983 | 549.5 | 0.026 | 0.999 |
| Adsorbent | Elovich | |||||
|---|---|---|---|---|---|---|
| α1 (mmol g−1 min−1) | β1 (g mmol−1) | R12 | α2 (mmol g−1 min−1) | β2 (g mmol−1) | R22 | |
| GO | 19.8 | 0.051 | 0.929 | 5.03 | 0.21 | 0.929 |
| GOKOH | 99.7 | 0.010 | 0.971 | 5.01 | 0.21 | 0.897 |
| Adsorbent | Intra-particle diffusion | |||||
|---|---|---|---|---|---|---|
| kd1 (g mg−1 min−0.5) | C1 (mg g−1) | R12 | kd2 (g mg−1 min−0.5) | C2 (mg g−1) | R22 | |
| GO | 3.7 | 57.4 | 0.868 | 0.35 | 109.8 | 0.947 |
| GOKOH | 25.2 | 181.1 | 0.917 | 0.43 | 513.1 | 0.853 |
The correlation coefficients R2 for the pseudo-second-order kinetic model of GO and GOKOH were higher than those for the pseudo-first-order kinetic model (shown in Fig. 11a and b). The calculated qe values resulted from the pseudo-second-order kinetic model were very close to the experimental data. Besides, the αi values were much higher than the βi values on each fitting plot from Elovich equation shown in Fig. 11c. These results indicate that the overall adsorption rates of orange IV onto GO and GOKOH were controlled by chemical adsorption.
The experimental data points were related by two straight lines in the intra-particle diffusion shown in Fig. 11d, corresponding to two different adsorption stages and two intra-particle diffusion rate constant kdi. The kdi values sharply decreased from kd1 to kd2, and the kdi values of GOKOH were more than 4 times as much as those of GO at each stage, consistent with the data shown in Fig. 7–9.
In addition, the two fitting plots with different kdi values indicated that the orange IV adsorption process onto GOKOH could be explained with the following portions: (i) the first stage corresponding to boundary layer diffusion and external surface adsorption; (ii) the second stage attributing to intra-particle diffusion with gradual equilibrium adsorption. So, for the removal of orange IV onto GOKOH, the intra-particle diffusion process might be another rate-limiting step.31,36
3.4. Adsorption isotherms
The adsorption isotherms determine how much adsorbents are required quantitatively for enrichment of an analyte from a given solution. The data can provide some physicochemical information on how the adsorption proceeds and how adsorbates interact with adsorbents.The experimental data of adsorption equilibrium were obtained by manipulating different initial orange IV concentrations of 50, 75, 100, 125, 150, 175 and 200 mg L−1. Adsorption time was kept constant at 24 hours to reach the equilibrium, and the data were analyzed with widely used Langmuir and Freundlich isotherms. The Langmuir empirical equations for isotherm models used are given below:
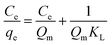 | (8) |
 | (9) |
The Freundlich isotherm model is an empirical model assuming a heterogeneous surface of adsorbent. The Freundlich isotherm is expressed as:
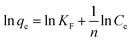 | (10) |
Isotherms studies afford the most important parameter for designing a desired adsorption system. The adsorption isotherms of orange IV onto the GO and GOKOH at different C0 concentrations are given in Fig. 12, and the data were fitted by Langmuir and Freundlich isotherm models.
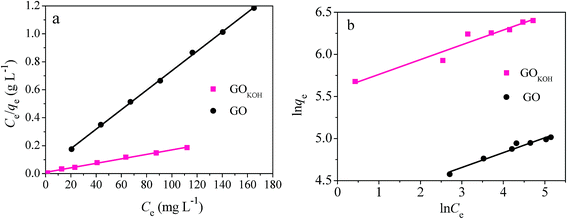 | ||
| Fig. 12 Langmuir (a) and Freundlich (b) isotherms for the adsorption of orange IV onto GO and GOKOH. Condition: ratio of solid to liquid of 0.15 g L−1, pH = 7, T = 298 K. | ||
Table 3 summarizes the Langmuir and Freundlich constants and the calculated coefficients. It can be found that the regression coefficient R2 obtained from Langmuir model was much higher than that from Freundlich model, suggesting that the Langmuir isotherm fitted better with the experimental data and the surface of GOKOH was monolayer covered with orange IV. However, it should be noticed that the R2 from Freundlich model was about 0.95, indicating that there was still few multilayer adsorption (not dominant) of orange IV onto GO and GOKOH.
| Adsorbent | Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (mg L−1) | R2 | RL | 1/n | KF (mg1−n Ln g−1) | R2 | |
| GO | 143.7 | 0.18 | 0.999 | 0.054 | 0.083 | 92.0 | 0.955 |
| GOKOH | 606.1 | 0.28 | 0.999 | 0.034 | 0.149 | 303.7 | 0.946 |
According to the Langmuir isotherm parameters, the qe from the adsorption of GOKOH for orange IV was 606.1 mg g−1 at 298 K, and was also much higher than that from GO of only 143.7 mg g−1. The result shows that the activation method with KOH is highly effective for GO.
For orange IV adsorption on GO and GOKOH, RL values obtained are much less than 0.1 in the range of 0.03–0.06, thereby confirming that the adsorption is a favorable process. The small n value (slightly larger than 1) suggested the high adsorption strength and chemical adsorption, which was consistent with the large KL value of the Langmuir model and indicated that orange IV was facile to be adsorbed onto GOKOH.
The larger parameters of k1, k2, KL and KF from kinetic and isotherm models of GOKOH, respectively, suggested that GOKOH adsorbent had faster adsorption rate and higher adsorption capacity for anionic dyes and surpassed the adsorption performances of many materials as shown in Table 4.
| Adsorbents | BET (m2 g−1) | Pollutants | Classification | Qm (mg g−1) | Ref. |
|---|---|---|---|---|---|
| a Standard Hummers method.b HTSTA method.c Modified Hummers method.d Staudenmaier method. | |||||
| GOa | 35.5 | Orange IV | Monoazo | 143.7 | This work |
| GOKOHb | 672.5 | Orange IV | Monoazo | 625.1 | This work |
| MCM-41 molecular sieve | 403 | Orange IV | Monoazo | 409.16 | 10 |
| Reduced GOc | — | Orange G | Monoazo | 5.98 | 35 |
| Magnetic GOa | — | Orange G | Monoazo | 20.85 | 38 |
| GOc | — | Reactive black 5 | Bisazo | 205 | 7 |
| GO/chitosanc | — | Remazol black | Bisazo | 277 | 3 |
| MCM-41 molecular sieve | 403 | Methyl orange | Monoazo | 366.58 | 10 |
| Activated carbon | 492 | Congo red | Monoazo | 300.0 | 4 |
| Iron oxide nanosphere | — | Reactive orange 13 | Monoazo | 32.5 | 9 |
| Montmorillonite-pillared GOc | 972 | Methyl orange | Monoazo | 145 | 39 |
| 3D-reduced GO | 303 | Acid red 1 | Monoazo | 277.01 | 40 |
| Magnetic graphene/chitosand | — | Acid orange 7 | Monoazo | 42.7 | 12 |
By means of HTSSA method with KOH as activating agent to improve the BET area and reduce the amount of oxygen-obtaining functional groups of GO, the large adsorption capacity and the rapid adsorption kinetics for anionic dyes were obtained, which strongly proposed a potential of GO-based structures to be versatile, promising and effective dye-pollutant adsorbent.
3.5. Adsorption thermodynamics
The thermodynamic studies provide in-depth information on inherent energetic changes that are associated with adsorption. To evaluate the effect of temperature influence on the adsorption and investigate the possible mechanism involved in the adsorption progress, three basic thermodynamic parameters, including the change of Gibbs free energy (ΔG0, kJ mol−1), enthalpy (ΔH0, kJ mol−1) and entropy (ΔS0, kJ mol−1 K−1) of adsorption were evaluated by the following equations:
ΔG0 = − RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (11) |
 | (12) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T) shown in Fig. 13.
Kd vs. 1/T) shown in Fig. 13.
| Adsorbent | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) | ΔG0 (kJ mol−1) | |||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | |||
| GO | 55.03 | 190.39 | −1.65 | −3.03 | −5.35 | −7.35 |
| GOKOH | 36.11 | 147.13 | −7.67 | −9.12 | −10.83 | −12.07 |
The positive values of ΔH0 calculated from the fitting line in Fig. 13 indicated an endothermic nature and were high enough to ensure the strong interaction between adsorbents and adsorbates, which was in accordant with the results of Fig. 10.
Generally, for the endothermic adsorption process, large and positive values of ΔH0 indicate that the adsorption of organic molecules on the surface of the solids involves chemical reactions, which may be due to hydrogen bond, chemical bond or protonation and so on.41,42 All the values of ΔG0 at four temperatures were negative, which indicated the feasibility of the process and spontaneous nature of the adsorption for orange IV onto GO and GOKOH. The calculated ΔG0 were very small values, indicating a strong interaction occurred between the adsorbed orange IV and adsorbents. And the Gibbs free energy has a small change (ΔG0 increases by about 2 kJ mol−1 when temperature increases by 10 °C), indicating that a steady adsorption took place between orange IV and adsorbents. Moreover, generally, when ΔG0 is less than −40 kJ mol−1, the adsorption involves chemisorption as analysed with FTIR.43
So, the results of positive values of ΔH0 and ΔS0 indicated that some structural changes may occur on the surface of GO and GOKOH surfaces, and the randomness at the solid–liquid interface was increased during the adsorption process.7,31,41 Thus, based on the data of adsorption isobar (Fig. 10), the FTIR data, the change of enthalpy and Gibbs free energy, and results of pseudo-second-order and Langmuir isotherm, the adsorption process of orange IV onto GO and GOKOH can be mainly attributed to chemisorption.
3.6. Desorption and recycling studies
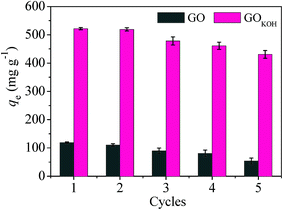
An excellent adsorbent should not only possess high adsorption capacity but also better reusability, which would significantly enhance the efficiency and reduce the overall cost of the adsorbent. Therefore, the desorption and regeneration experiments were carried out to study the reusability of GO and GOKOH and the results were presented in Fig. 14.The adsorption capabilities of the regenerated GO and GOKOH gradually decreased with increasing of the regeneration times. Compared with the first adsorption, the adsorption capabilities for orange IV with GO as adsorbents decreased by approximately 54.9% after five cycles. However, the decrease of adsorption capabilities for GOKOH was only about 17.5% after five cycles. This decrease can be attributed to loss of activated sites after each desorption step.25,27 The above results show that GOKOH could be used as a better adsorbent in the wastewater treatment applications.
3.7. Mechanism speculation
As a special material with huge BET area and super adsorption capacity, GOKOH has a lot of active sites effectively interacting with the dye molecules. A possible mechanism includes: (i) negatively charged oxygen-containing groups of hydroxyl (–OH) and carboxyl (–COOH) available to form the hydrogen bonds with the dye species.40,44 (ii) Ion-exchange from the hydroxyl (–OH) and carboxyl (–COOH) in GOKOH and R–SO3− (dissociated from orange IV, R represents the matrix of orange IV), resulting in the formation of R′′–C–O–SO2–R and R′′–COO–SO2–R (R′′ represents the matrix of GOKOH), respectively.However, the hydrogen bond and ion-exchange between orange IV molecules and oxygen-containing functional groups on the surface of GOKOH were not dominant, owing to the counteraction resulted from electrostatic repulsion between the negatively charged sulfonic groups (–SO3−) in orange IV and oxygen-containing functional groups in GOKOH. (iii) Weak electrostatic interactions and van der Waals forces.35 (iv) Combination of chemical bonds between sulfonic groups (R–SO3−) and GOKOH, resulting in the formation of C–O–S bond, based on the FTIR data. And the occurrence of the chemical bonds may be predominant during the adsorption of orange IV onto GOKOH.
4. Conclusions
In conclusion, the activated GO with highly efficient adsorption performance has been successfully prepared by means of a facile method of High Temperature Solid State Activation. The as-prepared GOKOH material had higher BET area of 672.5 m2 g−1 and less negatively charged oxygen-obtaining functional groups, which was favorable for the removal of anionic dye orange IV. The adsorption capacity of orange IV from the Langmuir isotherm model reached 606.1 mg g−1. Compared to many adsorbents, the GOKOH adsorbent exhibited extraordinary removal capacity and fast adsorption rate for anionic dye orange IV.The adsorption kinetics, isotherms and thermodynamics were investigated in detail. The kinetic study revealed that the adsorption process of orange IV followed the pseudo-second-order kinetic model. The equilibrium results were well fitted with the Langmuir isotherm model and the thermodynamic data indicated that the adsorption process was endothermic and spontaneous in nature.
Thus, the present investigation has provided a novel and promising method to improve the adsorption capacity of GO material for the adsorption of anionic dyes. And the as-obtained GOKOH can be used an excellent adsorbent with the value of practical application for water remediation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51578354), Natural Science Foundation of Jiangsu Province (No. BK20141179), the Practice Innovation Training Program Projects of the Jiangsu College Students (No. 201410332005Z), Suzhou Key Laboratory of Separation and Purification Materials & Technologies (SZS201512), Qing Lan Project and Overseas Training Program of the Outstanding Young Teachers and Principals of Universities of Jiangsu Provincial Department of Education.References
- V. Gari and M. Kim, Monatsh. Chem., 2015, 146, 1445 CrossRef.
- Z. H. Cheng, J. Liao, B. Z. He, F. Zhang, F. A. Zhang, X. H. Huang and L. Zhou, ACS Sustainable Chem. Eng., 2015, 3, 1677 CrossRef CAS.
- J. A. Gonzalez, M. E. Villanueva, L. L. Piehl and G. J. Copello, Chem. Eng. J., 2015, 280, 41 CrossRef CAS.
- M. K. Purkait, A. Maiti, S. DasGupta and S. De, J. Hazard. Mater., 2007, 145, 287 CrossRef CAS PubMed.
- C. Namasivayam and D. Kavitha, Dyes Pigm., 2002, 54, 47 CrossRef CAS.
- T. F. Jiao, Y. Z. Liu, Y. T. Wu, Q. R. Zhang, X. H. Yan, F. M. Gao, A. J. P. Bauer, J. Z. Liu, T. Y. Zeng and B. B. Li, Sci. Rep., 2015, 5, 10 Search PubMed.
- N. A. Travlou, G. Z. Kyzas, N. K. Lazaridis and E. A. Deliyanni, Chem. Eng. J., 2013, 217, 256 CrossRef CAS.
- L. L. Ji, Y. Shao, Z. Y. Xu, S. R. Zheng and D. Q. Zhu, Environ. Sci. Technol., 2010, 44, 6429 CrossRef CAS PubMed.
- M. Khosravi and S. Azizian, J. Ind. Eng. Chem., 2014, 20, 2561 CrossRef CAS.
- Q. D. Qin, J. Ma and K. Liu, J. Hazard. Mater., 2009, 162, 133 CrossRef CAS PubMed.
- V. V. Panic, S. I. Seslija, A. R. Nesic and S. J. Velickovic, Hem. Ind., 2013, 67, 881 CrossRef CAS.
- S. Sheshmani, A. Ashori and S. Hasanzadeh, Int. J. Biol. Macromol., 2014, 68, 218 CrossRef CAS PubMed.
- A. S. Patra, S. Ghorai, S. Ghosh, B. Mandal and S. Pal, J. Hazard. Mater., 2016, 301, 127 CrossRef CAS PubMed.
- X. Wu, D. Wu and R. Fu, J. Hazard. Mater., 2007, 147, 1028 CrossRef CAS PubMed.
- A. Gopalakrishnan, R. Krishnan, S. Thangavel, G. Venugopal and S. J. Kim, J. Ind. Eng. Chem., 2015, 30, 14 CrossRef CAS.
- S. Zamani and N. S. Tabrizi, Res. Chem. Intermed., 2015, 41, 7945 CrossRef CAS.
- S. X. Hu and Y. L. Hsieh, J. Mater. Chem. A, 2013, 1, 11279 CAS.
- Q. Jiang, M. Z. Qu, B. L. Zhang and Z. L. Yu, Carbon, 2002, 40, 2743 CrossRef CAS.
- S. H. Yoon, S. Lim, Y. Song, Y. Ota, W. M. Qiao, A. Tanaka and I. Mochida, Carbon, 2004, 42, 1723 CrossRef CAS.
- V. Barranco, M. A. Lillo-Rodenas, A. Linares-Solano, A. Oya, F. Pico, J. Ibanez, F. Agullo-Rueda, J. M. Amarilla and J. M. Rojo, J. Phys. Chem. C, 2010, 114, 10302 CAS.
- B. Zheng, T.-W. Chen, F.-N. Xiao, W.-J. Bao and X.-H. Xia, J. Solid State Electrochem., 2013, 17, 1809 CrossRef CAS.
- Y. W. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537 CrossRef CAS PubMed.
- B. J. Kim, Y. S. Lee and S. J. Park, J. Colloid Interface Sci., 2007, 306, 454 CrossRef CAS PubMed.
- E. Raymundo-Pinero, P. Azais, T. Cacciaguerra, D. Cazorla-Amoros, A. Linares-Solano and F. Beguin, Carbon, 2005, 43, 786 CrossRef CAS.
- Y. B. Song, X. D. Song, C. J. Cheng and Z. G. Zhao, RSC Adv., 2015, 5, 87030 RSC.
- J. N. Tiwari, K. Mahesh, N. H. Le, K. C. Kemp, R. Timilsina, R. N. Tiwari and K. S. Kim, Carbon, 2013, 56, 173 CrossRef CAS.
- P. Tan, J. Sun, Y. Hu, Z. Fang, Q. Bi, Y. Chen and J. Cheng, J. Hazard. Mater., 2015, 297, 251 CrossRef CAS PubMed.
- H. M. Khan, M. Ashraf, A. Hashmi, M. U. D. Ahmad and A. A. Anjum, Pak. Vet. J., 2013, 33, 471 CAS.
- Y. Fan, W. Wang, W. Song, H. Chen, A. Teng and A. Liu, Carbohydr. Polym., 2012, 88, 1313 CrossRef CAS.
- M. Zhu, X. M. Li, Y. B. Guo, X. Li, P. Z. Sun, X. B. Zang, K. L. Wang, M. L. Zhong, D. H. Wu and H. W. Zhu, Nanoscale, 2014, 6, 4909 RSC.
- L. Cui, Y. Wang, L. Hu, L. Gao, B. Du and Q. Wei, RSC Adv., 2015, 5, 9759 RSC.
- X. X. Yang, Y. H. Li, Q. J. Du, J. K. Sun, L. Chen, S. Hu, Z. H. Wang, Y. Z. Xia and L. H. Xia, J. Colloid Interface Sci., 2015, 453, 107 CrossRef CAS PubMed.
- R. W. Sabnis, Handbook of Acid-Base Indicators, CRC Press, 2007 Search PubMed.
- I. Laaz, M. J. Stebe, A. Benhamou, D. Zoubir and J. L. Blin, Colloids Surf., A, 2016, 490, 30 CrossRef CAS.
- G. K. Ramesha, A. V. Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270 CrossRef CAS PubMed.
- J. H. Chen, Q. L. Liu, S. R. Hu, J. C. Ni and Y. S. He, Chem. Eng. J., 2011, 173, 511 CrossRef CAS.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2 CrossRef CAS.
- J. H. Deng, X. R. Zhang, G. M. Zeng, J. L. Gong, Q. Y. Niu and J. Liang, Chem. Eng. J., 2013, 226, 189 CrossRef CAS.
- L. Liu, B. Zhang, Y. Zhang, Y. He, L. Huang, S. Tan and X. Cai, J. Chem. Eng. Data, 2015, 60, 1270 CrossRef CAS.
- H. Kim, S.-O. Kang, S. Park and H. S. Park, J. Ind. Eng. Chem., 2015, 21, 1191 CrossRef CAS.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo and W. S. Guo, Sci. Total Environ., 2015, 532, 112 CrossRef CAS PubMed.
- H. R. Pouretedal and N. Sadegh, J. Water Process Eng., 2014, 1, 64 CrossRef.
- L. Yu, Z. Liu, W. Yang and Z. Wang, Curr. Anal. Chem., 2016, 12, 141 CrossRef CAS.
- A. Roy, B. Adhikari and S. B. Majumder, Ind. Eng. Chem. Res., 2013, 52, 6502 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |