A remarkable chiral recognition of racemic Mosher's acid salt by naturally derived chiral ionic liquids using 19F NMR spectroscopy†
Abstract
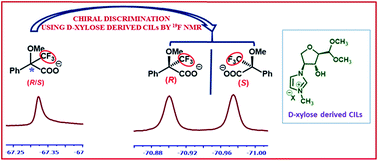
A new class of D-xylose derived imidazolium-based chiral ionic liquids were designed and synthesized via simple tuning approaches. The developed chiral ionic liquids were tested for their chiral recognition properties with racemic Mosher's acid salt using 19F NMR spectroscopy. For the first time, we demonstrate the excellent enantioselective discrimination of the racemic salt using significantly less equivalents of carbohydrate derived chiral ionic liquids. We have determined the enantiomeric excess values of non-racemic mixtures of Mosher's acid salts using 19F NMR spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...