Study on a hydrophobic Ti-doped hydroxyapatite coating for corrosion protection of a titanium based alloy
Abstract
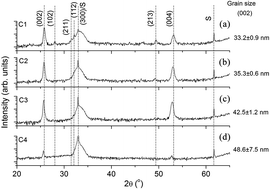
In the paper, hydroxyapatite coatings enriched with Ti were prepared as a possible candidate for biomedical applications, especially for implantable devices that are in direct contact with bone. The hydroxyapatite coatings with different Ti contents were prepared by an RF magnetron sputtering method on a Ti6Al4V alloy using pure hydroxyapatite and TiO2 targets. The Ti content was modified by changing the RF power fed to the TiO2 target. The formation of the hydroxyapatite compound was not influenced by the addition of Ti. The Ca/P ratio of the Ti-doped hydroxyapatite coatings was found to be in the range between 1.64 and 1.68, which is close to the stoichiometric hydroxyapatite coating. The roughness of the doped hydroxyapatite coatings was augmented by increasing the RF power on the TiO2 cathode. The addition of Ti led to an increase in the contact angle of the hydroxyapatite coatings. The in vitro corrosion performance of the Ti6Al4 alloy was improved significantly by the hydrophobic hydroxyapatite coatings with and without the Ti addition. A surface with a higher Ti concentration and water contact angle exhibited a better corrosion resistance in simulated body fluid at 37 °C. Therefore, the deposition of a hydrophobic Ti-doped HA coating could be a promising surface treatment for the improvement of the electrochemical behaviour of metallic implants.

 Please wait while we load your content...
Please wait while we load your content...