Lamellar supramolecular materials based on a chelated metal complex for organic dye adsorption†
Yanan Zhanga,
Yun Yan*b,
Jide Wang*a and
Jianbin Huang*ab
aCollege of Chemistry and Chemical Engineering, Xinjiang University, Urumqi, 830046, China
bBeijing National Laboratory for Molecular Sciences (BNLMS), State Key Laboratory for Structural Chemistry of Unstable and Stable Species, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: yunyan@pku.edu.cn; awangjd@126.com; jbhuang@pku.edu.cn
First published on 29th March 2016
Abstract
Poisonous industrial wastes have become the major threat to people's health. Recycling these components and making them into useful materials is needed urgently. We report that with the assistance of dodecylamine (DA), the chelating metal pollutant Cu-EDTA can be made into the lamellar Cu-EDTA@2DA supramolecular material. Since Cu-EDTA is notorious for its highly solubility and stability in water, this work for the first time showed that it can be made into supramolecular material which precipitated out from water. Furthermore, this Cu-EDTA@2DA supramolecular material shows excellent efficiency in adsorbing organic dyes, and can be recycled upon desorption of dyes in N,N-dimethylformamide (DMF). As far as we know, this is the first case of using industrial waste to build well-defined useful supramolecular materials. We expect that the strategy of ‘fight poison with poison’ can be an effective and economic approach to treat environmental pollution.
Introduction
Molecular self-assembly has become a very efficient approach to construct various functional materials,1–5 among which novel building blocks6–10 play crucial roles in the formation of diverse self-assembled structures, and considerable attention has been paid to synthetic chemistry or supramolecular chemistry to create desired self-assembled materials.1,4,11,12 However, so far little effort has been made to employ industrial waste in the fabrication of molecular self-assemblies. It is well-known that there are many chemicals in industrial waste water and most of them jeopardize and threaten the safety of ecological systems and the health of human beings due to serious environmental pollution.13–16 Therefore, building self-assembled materials using industrial wastes is highly desired both from the view of environmental protection and recycle of chemicals.Among various industrial wastes and pollutants, heavy metals jeopardize serious damage to people's health. Usually, precipitation method can effectively remove free metal ions from water,14,17 but those in chelating states, are hardly be precipitated14,18 and are not biodegradable so that can accumulate in living organisms to exert permanent harm to human beings.19,20 It is so difficult to remove chelating metals from water that many countries and areas have to forbidden the use of metal plating technique, where solution containing ethylenediaminetetraacetate (EDTA), or other chelating agents were used to wash the final product to remove the excess metal ions by the formation of metal-EDTA chelating complexes.18,21 Therefore, building self-assembled materials using chelating metal complexes not only can help to solve environmental pollution, but also an issue to save the metal plating industry.
Herein we report that upon employment the strategy of molecular self-assembly, the Cu-EDTA chelating complexes can be made into lamellar supramolecular materials which precipitate out of water. In our strategy, dodecylamine (DA) was employed to bind with Cu-EDTA to form the Cu-EDTA@2DA supramolecules, which further self-assemble into giant lamellar structure and precipitate out of water. It is striking that this Cu-EDTA@2DA supramolecular material displays very high efficiency to adsorb organic dyes. Since organic dyes are a family of robust environmental pollutant which display toxicity and carcinogenic effect for aquatic organisms,22 a lot of efforts were made specifically to remove them from water. To this end, various supramolecular systems have been designed.23–26 In contrast to literature results, our work for the first time demonstrates the success of building dye adsorbents with the self-assembled materials based on chelating metal complexes, which on their own are notorious pollutants. Most significantly, the dyes can be desorbed from the Cu-EDTA@2DA supramolecular material simply by immersion into N,N-dimethylformamide (DMF), which manifests that both the Cu-EDTA@2DA supramolecular material and the dyes can be recycled. We expect that this work may open a new vista for the practical application of molecular self-assembly technique.
Materials and methods
Materials
Dodecylamine (DA) was from SCRC, ethylenediaminetetraacetate acid disodium salt (EDTA-2Na) and copper(II) chloride dehydrate (CuCl2·2H2O) were from Beijing Chemical Company. Acid chrome blue K (ACBK) was purchased from SCRC. Azo Rubine (AR) and Sunset Yellow (SY) were from TCI. Susan III and Rhodamine B (RB) were from Beijing Chemical Company. The chemical structures of the dyes are shown in Fig. 1. All chemicals were of A.R. grade and were used as received.Preparation of layered materials
The supramolecular materials of Cu-EDTA@2DA were prepared as follow: 0.3724 g of Na2EDTA·2H2O, 0.1704 g of CuCl2·2H2O and 0.5550 g of dodecylamine were dissolved in 67 g of water. The resulting mixture was stirred at 45 °C for 2 h and finally left at room temperature. After 48 h, blue precipitates were found at the bottom of the Erlenmeyer flask. The precipitates were separated, washed with distilled water for several times, and dried in the vacuum oven at room temperature for 24 h for further analysis.Characterization
Scanning electron microcopy (SEM) measurements were made on Hitachi S4800 operated at 10 kV. Elemental analysis was carried out with the Elementar Vario MICRO CUBE (Germany). Powder X-ray diffraction (XRD) patterns were measured using a Rigaku Dmax-2400 diffractometer with Cu Kα radiation. Ultraviolet-visible (UV-vis) spectral measurements were performed on a Shimadzu UV-1800 spectrophotometer. The concentration of Cu2+ in liquid phase was determined by Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP-AES) (PROFILE SPEC, Leeman). Fourier transform infrared (FT-IR) spectra were recorded with a Bruker Vector-22 spectrophotometer. For FT-IR measurements, the dry powders of the Cu-EDTA@2DA samples were pressed onto the KBr pellet. X-ray photoelectron spectroscopy (XPS) spectra were recorded on an AXIS-Ultra Imaging Photoelectron Spectrometer from Kratos Analytical Ltd, using monochromatic Al-Kα radiation in a vacuum of 2 × 10−8 Pa. Zeta potentials were obtained with a temperature-controlled ZetaPALS Zeta Potential Analyzer.Adsorption experiments
Adsorption of dye from aqueous solutions was examined with UV-vis spectrophotometer. A standard work curve of dyes was obtained firstly, and the removal efficiency of dyes can be calculated according to the following equation:where C0 and Ce are the initial and equilibrium dye concentrations (mg L−1), respectively.
Results and discussion
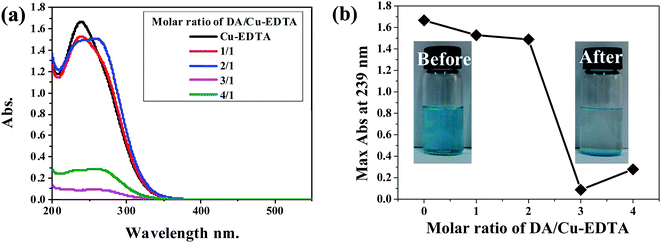
Cu-EDTA is one of the notorious industrial wastes produced in copper-splatting industry, since it is very stable in water and can hardly be precipitated. However, we found that upon addition of DA into the aqueous solution of Cu-EDTA, blue precipitates occur immediately. Fig. 2 shows the drop of the UV-vis absorption of the solution with varying the ratio between Cu-EDTA and DA. The absorption decreases to the minimum at the DA/Cu-EDTA molar ratio of 3/1. ICP measurements suggested that around 64% Cu-EDTA have been precipitated.Amazingly, SEM observation (Fig. 3) revealed that the blue precipitates are perfect lamellar materials. FT-IR measurements (Fig. 4) suggested the coexisting of EDTA and DA in the precipitates. It is clear in Fig. 4 that the characteristic vibration features for both EDTA and DA are observed in the precipitates. Detailed analysis of the FT-IR spectra can be found in the following text. Meanwhile, the ratio of Cu-EDTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DA was found to be 1
DA was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with elemental analysis. This means that every Cu-EDTA chelating complex bind with two DA molecules to form the Cu-ETDA@2DA supramolecule in the lamellar materials. XRD measurements in Fig. 5a display sharp peaks featuring the 001, 002, 003, 004, and 005 diffractions. The distance corresponding to the 001 diffraction is 2.53 nm, which is in between one and two folds of the extending length of DA (1.9 nm),28 suggesting the presence of highly ordered interdigitated bilayers of DA (ref. 28–30) or tilled DA bilayers with the tilting angle of 42°,31–34 as illustrated in Fig. 5b and c. The presence of DA bilayers can also be inferred from the FT-IR spectra in Fig. 4, supported by the two strong sharp bands at 2850 and 2920 cm−1, which features the symmetric and asymmetric vibration of the highly ordered alkyl chains in DA.42,43 This means that the formation of the lamellar structure is driven by the hydrophobic effect of the Cu-ETDA@2DA supramolecule, which behaves just as conventional amphiphiles. It is possible that double tail structure of Cu-ETDA@2DA allows the packing parameter close to 1, so that they self-assemble into lamellar structures.27
2 with elemental analysis. This means that every Cu-EDTA chelating complex bind with two DA molecules to form the Cu-ETDA@2DA supramolecule in the lamellar materials. XRD measurements in Fig. 5a display sharp peaks featuring the 001, 002, 003, 004, and 005 diffractions. The distance corresponding to the 001 diffraction is 2.53 nm, which is in between one and two folds of the extending length of DA (1.9 nm),28 suggesting the presence of highly ordered interdigitated bilayers of DA (ref. 28–30) or tilled DA bilayers with the tilting angle of 42°,31–34 as illustrated in Fig. 5b and c. The presence of DA bilayers can also be inferred from the FT-IR spectra in Fig. 4, supported by the two strong sharp bands at 2850 and 2920 cm−1, which features the symmetric and asymmetric vibration of the highly ordered alkyl chains in DA.42,43 This means that the formation of the lamellar structure is driven by the hydrophobic effect of the Cu-ETDA@2DA supramolecule, which behaves just as conventional amphiphiles. It is possible that double tail structure of Cu-ETDA@2DA allows the packing parameter close to 1, so that they self-assemble into lamellar structures.27
 | ||
| Fig. 5 (a) XRD patterns of the supramolecular material of Cu-EDTA@2DA. (b) and (c) are the schematic illustration of the possible molecular packing in the lamellar material of Cu-EDTA@2DA. | ||
The formation of perfect supramolecular materials of Cu-EDTA@2DA which can precipitates from water is very amazing. On the one hand, so far it still remains challenging to precipitate Cu-EDTA from water, since they are very stable against strong base, acid, and oxidant. A lot of cost efforts were made to liberate copper from its complex with EDTA.35–38 However, our work shows that upon using the strategy of supramolecular self-assembly, the Cu-EDTA complex can be precipitated as a whole from water, which greatly lowered the energy cost and made it possible to put into practical applications in water treatment. On the other hand, the presence of alternative hydrophilic and hydrophobic domains in the perfect lamellar precipitates of Cu-EDTA@2DA enabled them being adsorbents for a number of organic pollutants. Fig. 6a–c shows that the UV-vis absorption of three anionic dyes, Acid Chrome Blue K (ACBK), Azo Rubine (AR), and Sunset Yellow (SY), reduced drastically upon addition of the layered materials. Similarly, the UV-vis absorption of charge neutral dyes, such as Sudan III, was also found declined slowly with addition of the Cu-ETDA@2DA layered material (Fig. 6d), which indicated that the layered material was capable of removing oil-soluble dyes, too. However, the material shows negligible adsorption toward cationic dyes, such as Rhodamine B (RB), (Fig. 6e). This means that the absorption of these ionic dyes is mainly via electrostatic and hydrophobic interaction.
Indeed, zeta potential measurements revealed the surface electrical potential for the blue precipitates being 20.1 ± 2 mV. FT-IR measurements (Fig. 4) suggest that the positive charge is originated from the –NH3+ group, which can be verified by the asymmetric and symmetric deflection of –NH3+ groups at 1626 cm−1 and 1538 cm−1 in the FT-IR spectra.32,39 Meanwhile, there are also weak bands at 1597 cm−1 in the FT-IR spectra, which belongs to the bending motion of –NH2 group.32,40 The coexistence of –NH3+ and –NH2 groups was further verified with the XPS measurement. As is shown in Fig. 7, both Cu-EDTA and Cu-EDTA@2DA displays two peaks around 399.7 and 401.4 eV, which are the N1s energy corresponding to –NH2 and –NH3+,32,41 respectively. However, it is noticed that in the Cu-EDTA@2DA system, the fraction of –NH3+ is 46.03%, which is significantly higher than the fraction of 27% for the free Cu-EDTA. This means that the binding of DA to the Cu-EDTA coordinating complex have produced more cationic charges, which generates electrostatic attraction to remove anionic dyes from water. As to the removal of charge neutral dyes, it can be attributed to the solvation of the dyes in the hydrophobic domains formed by the long alkyl chains of DA.
 | ||
| Fig. 7 XPS measurements for N1s electrons of (a) Cu-EDTA and (b) Cu-EDTA@2DA supramolecular materials. | ||
The adsorption of dyes didn't change the layer spacing after adsorption of the anionic dyes ACBK, AR and SY (Fig. 8), suggesting the presence of large separations between the two adjacent bilayers which is probably required by the repulsive forces between them. Upon adsorption of negatively charged dyes, the dyes can just be hosted in between the bilayer spacing. However, as to the adsorption of neutral dye Sudan III, the dye molecules have to squeeze into the bilayers, so that the bilayer has to rearrange to form close stacking. As a result, the interlayer spacing decreased from 2.53 nm to 2.29 nm after adsorption of Sudan III (Fig. 8).
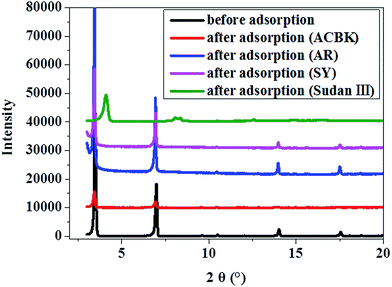 | ||
| Fig. 8 XRD patterns for the supramolecular materials of Cu-EDTA@2DA before and after the adsorption of various dyes. | ||
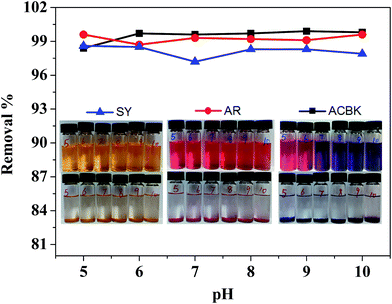
Significantly, the Cu-EDTA@2DA supramolecular materials display broad pH windows for the dye removal. Fig. 9 shows that the removal efficiency for these dyes is hardly affected by changing pH in the pH range of 5–10, suggesting they are indeed potent adsorbing materials that can be used in wide range of industrial polluted water system.
Regeneration of the adsorbent is an important factor in practical application, especially in the ‘green’ protocol of pollution treatment. Desorption of the dyes can be achieved by addition of DMF to the dye-saturated precipitates. Fig. 10a shows that upon addition of DMF to the dark purple ACBK/Cu-EDT@2DA precipitates, the supernatant becomes purple and the precipitate recover its original blue color. Most interestingly, this blue precipitate display a high adsorption efficiency of over 99% even after three cycles (Fig. 10b), which indicates that the layered materials could be regenerated. Similarly, other dyes, such as AR, SY, and Sudan III were also find desorbed from the Cu-EDT@2DA in DMF. It is should be mentioned that there are also release of Cu-EDTA to the water as the dyes were adsorbed by the Cu-EDTA@2DA lamellar materials, and the releasing rate is about 36% determined with (ICP-AES). However, this released Cu-EDTA can be further precipitated cooperatively by addition of a cationic polyelectrolyte polyethyleneamine (PEI) and sodium dodecyl sulphate (SDS), and the final copper concentration can be lowered to 1.79 mg L−1. Systematic analysis will be reported in a forthcoming work.
Conclusion
The water pollutant industrial waste Cu-EDTA can be employed to form supramolecular materials with the assistance of dodecylamine (DA) in the form of lamellar precipitates. This lamellar material has both positive charges and hydrophobic domains which are capable of adsorb both anionic and neutral organic dyes. The adsorbed dyes can be desorbed in DMF. In this way, both the dyes and the Cu-EDTA@2DA supramolecular materials can be recycled. This work for the first time realized the precipitation of Cu-EDTA as a supramolecular material, which demonstrated an economic strategy of ‘Fight poison with poison’ in the field of environmental science.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (NSFC 21573011, 21422302).References
- L. C. Palmer and S. I. Stupp, Acc. Chem. Res., 2008, 41, 1674–1684 CrossRef CAS PubMed.
- S. Zhang, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS PubMed.
- K. Rajagopal and J. P. Schneider, Curr. Opin. Struct. Biol., 2004, 14, 480–486 CrossRef CAS PubMed.
- S. Chen, P. Slattum, C. Wang and L. Zang, Chem. Rev., 2015, 115, 11967–11998 CrossRef CAS PubMed.
- P. Zhou, R. Shi, J.-f. Yao, C.-f. Sheng and H. Li, Coord. Chem. Rev., 2015, 292, 107–143 CrossRef CAS.
- L. Zang, Y. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596–1608 CrossRef CAS PubMed.
- J. F. Li, Z. J. Chen, M. C. Zhou, J. B. Jing, W. Li, Y. Wang, L. X. Wu, L. Y. Wang, Y. Q. Wang and M. Lee, Angew. Chem., Int. Ed., 2016, 55, 2592–2595 CrossRef CAS PubMed.
- J. Elemans, R. van Hameren, R. J. M. Nolte and A. E. Rowan, Adv. Mater., 2006, 18, 1251–1266 CrossRef CAS.
- T. Li, M. Smet, W. Dehaen and H. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 3609–3614 CAS.
- K. Liu, R. Xing, C. Chen, G. Shen, L. Yan, Q. Zou, G. Ma, H. Mohwald and X. Yan, Angew. Chem., 2015, 54, 500–505 CAS.
- O. A. Bell, G. Wu, J. S. Haataja, F. Brommel, N. Fey, A. M. Seddon, R. L. Harniman, R. M. Richardson, O. Ikkala, X. Zhang and C. F. Faul, J. Am. Chem. Soc., 2015, 137, 14288–14294 CrossRef CAS PubMed.
- S. I. Stupp, V. LeBonheur, K. Walker, L. S. Li, K. E. Huggins, M. Keser and A. Amstutz, Science, 1997, 276, 384–389 CrossRef CAS PubMed.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- X. Ying and Z. Fang, J. Hazard. Mater., 2006, 137, 1636–1642 CrossRef CAS PubMed.
- A. Saeed, M. Sharif and M. Iqbal, J. Hazard. Mater., 2010, 179, 564–572 CrossRef CAS PubMed.
- C. A. Martínez-Huitle and E. Brillas, Appl. Catal., B, 2009, 87, 105–145 CrossRef.
- Y. K. Chang, J. E. Chang, T. T. Lin and Y. M. Hsu, J. Hazard. Mater., 2002, 94, 89–99 CrossRef CAS PubMed.
- W. Msketion, C. Z. Zenner and K. L. Ogden, Environ. Sci. Technol., 2008, 42, 2124–2129 CrossRef.
- U. Garg, M. P. Kaur, G. K. Jawa, D. Sud and V. K. Garg, J. Hazard. Mater., 2008, 154, 1149–1157 CrossRef CAS PubMed.
- J. Lu, Y. Li, M. Yin, X. Ma and S. Lin, Chem. Eng. J., 2015, 267, 86–92 CrossRef CAS.
- S. Jiang, F. Fu, J. Qu and Y. Xiong, Chemosphere, 2008, 73, 785–790 CrossRef CAS PubMed.
- E. Lorencgrabowska and G. Gryglewicz, Dyes Pigm., 2007, 74, 34–40 CrossRef CAS.
- X. Qin, G. Zhang, Y. Gao, H. Liu, C. Du and Z. Liu, RSC Adv., 2015, 5, 43334–43337 RSC.
- L. Xiao, Y. Xiong, S. Tian, C. He, Q. Su and Z. Wen, Chem. Eng. J., 2015, 265, 157–163 CrossRef CAS.
- B. O. Okesola and D. K. Smith, Chem. Commun., 2013, 49, 11164–11166 RSC.
- M. Chen, W. Ding, J. Wang and G. Diao, Ind. Eng. Chem. Res., 2013, 52, 2403–2411 CrossRef CAS.
- Y. Zhao, J. Zhao, Y. Yan, Z. Li and J. Huang, Soft Matter, 2010, 6, 3282 RSC.
- J. Wang, Z. H. Liu, X. Tang and K. Ooi, J. Colloid Interface Sci., 2007, 307, 524–530 CrossRef CAS PubMed.
- J. Q. Li, B. Marler, H. Kessler, M. Soulard and S. Kallus, Inorg. Chem., 1997, 36, 4697–4701 CrossRef CAS PubMed.
- M. Pospíšil, A. Kalendová, P. Čapková, J. Šimoník and M. Valášková, J. Colloid Interface Sci., 2004, 277, 154–161 CrossRef PubMed.
- G. Alerti, F. Marmottini, S. Cavalaglio and D. Severi, Langmuir, 2000, 16, 4165–4170 CrossRef.
- J. J. Benítez, M. A. San-Miguel, S. Domínguez-Meister, J. A. Heredia-Guerrero and M. Salmeron, J. Phys. Chem. C, 2011, 115, 19716–19723 Search PubMed.
- T. Nakamura and M. Ogawa, Langmuir, 2012, 28, 7505–7511 CrossRef CAS PubMed.
- A. Matsumoto, S. Oshita and D. Fujioka, J. Am. Chem. Soc., 2002, 124, 13749–13756 CrossRef CAS PubMed.
- X. Zhao, L. Guo and J. Qu, Chem. Eng. J., 2014, 239, 53–59 CrossRef CAS.
- K. A. Sashkina, A. V. Polukhin, V. S. Labko, A. B. Ayupov, A. I. Lysikov and E. V. Parkhomchuk, Appl. Catal., B, 2016, 185, 353–361 CrossRef CAS.
- S. S. Lee, H. Bai, Z. Liu and D. D. Sun, Environ. Sci. Technol., 2015, 49, 2541–2548 CrossRef CAS PubMed.
- F. H. Wang, Y. X. Ji and J. J. Wang, J. Hazard. Mater., 2012, 241–242, 427–432 CrossRef CAS PubMed.
- Y. Komori, Y. Sugahara and K. Kuroda, Appl. Clay Sci., 1999, 15, 241–252 CrossRef CAS.
- S. Zhang, Q. Liu, H. Cheng, X. Li, F. Zeng and R. L. Frost, J. Colloid Interface Sci., 2014, 430, 345–350 CrossRef CAS PubMed.
- Y. Matsuo, T. Miyabe, T. Fukutsuka and Y. Sugie, Carbon, 2007, 45, 1005–1012 CrossRef CAS.
- Y. Yan, S. Guo, W. Xiong, J. B. Huang, Z. C. Li and J. M. Ma, Acta Phys.–Chim. Sin., 2005, 21, 550–555 CAS.
- A. Badia, L. Cuccia, L. Demers, F. Morin and R. B. Lennox, J. Am. Chem. Soc., 1997, 119, 2682–2692 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed elemental analysis process was given in the ESI. See DOI: 10.1039/c6ra03381d |
| This journal is © The Royal Society of Chemistry 2016 |