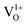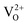Why host to dopant energy transfer is absent in the MgAl2O4:Eu3+ spinel? And exploring Eu3+ site distribution and local symmetry through its photoluminescence: interplay of experiment and theory†
Santosh K. Gupta*a,
P. S. Ghoshb,
Nimai Pathaka and
R. M. Kadama
aRadiochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India. E-mail: santoshg@barc.gov.in; santufrnd@gmail.com; Fax: +91-22-25505151; Tel: +91-22-25590636
bMaterials Science Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400085, India
First published on 25th April 2016
Abstract
An undoped and Eu3+ doped magnesium aluminate spinel (MAS) was synthesized using a citric acid assisted combustion technique. MAS samples were characterized systematically using X-ray diffraction (XRD), time resolved photoluminescence spectroscopy (TRPLS) and ab initio calculations. On irradiating the undoped MAS with UV light; multicolor emission is observed. The blue emission peak was attributed to Mg2+ vacancies whereas the one in the green region was attributed to oxygen vacancies. Based on the emission spectrum it was inferred that the majority of europium ions are localized at the Mg2+ site which was also confirmed using lifetime measurements. DFT based cohesive energy calculations also showed Eu doping in the Mg position is energetically more favorable than doping in the Al position. Photoluminescence (PL) spectroscopy shows that the emission spectrum consists of host as well as Eu3+ emission indicating the absence of complete host–dopant energy transfer. DFT calculated density of states analysis shows that Eu states are solely localized in VB and CB regions and do not contribute in defects states. From the emission spectrum of the undoped MAS sample it was observed that photo-luminescence properties of the MgAl2O4 are dominantly governed by the defect states coming from the presence of cation and oxygen vacancies (neutral and charged). As a result photon energy transfer from host MAS to dopant Eu is difficult. The actual site symmetry for europium ions in MAS was also evaluated based on a stark splitting pattern which comes out to be C2v. Based on Judd–Ofelt analysis it was found that the Ω2 value is greater than Ω4; indicating high covalency and low symmetry around europium ions which is also observed in the emission spectrum. The high purity of the red emission coupled with good fluorescence quantum yields highlights the potential of this unexplored MAS as a promising phosphor.
1. Introduction
It is known that the photophysical properties of lanthanide ions are highly sensitive to their immediate coordinating environment, and the emission intensity of Ln3+-doped materials is critically dependent on the crystal structure and crystal field of the surrounding Ln3+ emitters. A slight change in the local structure will bring about a substantial change in the optical characteristics of Ln3+ doped materials. Structural information (local site, point group symmetry etc.) of luminescence material is very essential for optimizing their optical performance as well as exploring novel host materials for efficient photoluminescence and upconversion phenomena. Such information can only be revealed from XRD at very high dopant ion concentrations which may affect the performance of the luminescent material through concentration quenching. Among all the Ln3+ ions, the Eu3+ ion is considered to be the most extensively used spectroscopic probe because of its non-degenerate emissive (5D0) and ground state of 7F0.1 The 5D0 → 7F2 transition of Eu3+ is of an electric-dipole (ED) origin is hypersensitive to its local site symmetry whereas the 5D0 → 7F1 transition is of a magnetic-dipole (MD) origin and insensitive to the local site symmetry.2 The symmetry around the lanthanide ion can thus be obtained from the shape of the emission spectrum of the Eu3+ ion.Recently, by means of low temperature Eu3+ photoluminescence, breakdown in crystallographic site symmetry of NaYF4 (both α and β phase) and KGdF4 on Eu3+ doping has been studied.3 It is easy to investigate size and structure sensitive optical characterisation using Eu3+ ion as a spectroscopic probe. Recently our group at Bhabha Atomic Research Centre have used Eu3+ as spectroscopic probe to investigate the effect of particle size and structural changes on the optical properties of yttrium indium oxide, YInO3/SrWO4 (ref. 4) and thorium oxalate hydrate5 respectively. Europium is also used as a probe to investigate the difference in energy transfer dynamics of CaMoO4 and SrMoO4 scheelite6 and also to probe the crystallographic site swapping of La3+ ion in BaA′LaTeO6 (A′ = Na, K, Rb) double perovskite.7 J. S. Yu and group have found europium ion occupied a highly symmetric site in AgLa(PO3)4 whereas site with non-inversion symmetry in SrWO4.8,9
It has also been used as probe to monitor temperature induce phase transition in CeZrO2.10 It was found to be an excellent probe for surface versus inner lattice sites location, Eu–defects interactions, nanoscale homogeneity and defects configuration under fuel cell operating conditions in CeO2 nanoparticle.11 Not only europium ion is a good spectroscopic probe but it is an efficient red emitter.12
An AB2O4 spinel structure exists in various phases exhibiting interesting physiochemical properties and can be a potential host for chemical doping. Because of various interesting properties spinels are used extensively for various technological application such as Li ion battery,13 magnetism,14 catalysis,15 temperature sensor,16 drug delivery17 etc. The interesting fact about spinel structures is that it can accommodate dopant on both A-site or B-site, and thereby exploring suitable experimental techniques one can probe not only local environment in these doped oxide but also induce significant changes in their functional properties like optical, magnetic, catalytic etc. Recently, magnesium aluminate spinel (MAS) MgAl2O4 has received a considerable interest both from academic as well as industrial application point of view because of its suitable and advantageous properties such as high melting point (2135 °C), high hardness (16 GPa), relatively low density (3.58 g cm−3), high mechanical strength both at room (135–216 MPa) and elevated temperatures (120–205 MPa at 1300 °C), high resistance to chemical attack and thermal shock, wide band gap, high electrical resistivity, relatively low thermal expansion coefficient (9 × 10−6 °C−1 between 30 and 1400 °C).18 It is considered as one of the most promising advanced ceramic materials and has been explored for various technological applications in light emitting diode,19 luminescence host,20 catalysis,21 magnetism,22 potential core material in fusion reactor, electronic humidity sensor, integrated electronic devices, electrode materials, catalysis etc.18
Sickafus et al.23 long back has proposed a clear picture about the structure of spinel. In particular MAS crystallizes into a face centred cubic structure with Fd3m space group and the unit cell consisting 64 tetrahedral and 32 octahedral sites to be occupied by 8Mg2+ and 16Al3+ ions respectively, which corresponds to eight units per cubic cell [(Mg)8(Al)16O32]. In an ideal MAS spinel structure Mg2+ are present in tetrahedral voids and the Al3+ in octahedral voids respectively. However in general some percentage of Mg2+ in octahedral sites and Al3+ in tetrahedral sites are generally observed, which leads to inverse spinel structure with general formula (Mg1−xAlx)tetra[MgxAl2−x]octaO4, where x stand for inversion parameter and it defined as the fraction of Al3+ in tetrahedral sites. For ideal spinel, x = 0 and for inverse spinel, x = 1 while in mixed spinel, 0 < x < 1.
As far as europium doped MAS is concerned there are quite a few reports in literature but none of them clearly explained the local site occupancy of Eu3+ and its point group symmetry.
Europium-doped MAS provides a promising red emitting phosphor for light emitting diodes (LEDs). Chen et al.24,25 has hydrothermally synthesized Eu doped MAS in different morphology such as plates, spheres, rods and demonstrated their application as red phosphor. Omkaram et al. has used simple solid state reaction to synthesized Eu3+ doped MAS and mechanism involved in the generation of red emission has also been explained.26 Wiglusz and group27 have suggested that Eu3+ ions mainly replace Mg2+ in the MAS host lattice and this attribution is based solely on the fact that 5D0–7F2 transition band is dominant without justifying with any other technique. Similar hypothesis were also suggested by Guan et al.28 where they have proposed that part of the Eu3+ ions mainly replace Mg2+ ions in the spinel structure and the remaining residue on the surface of MgAl2O4. There are few works where authors have optimized the size of the phosphor particle, dopant concentration as well as annealing condition for best red emission output with high color purity.29–31 But still in all these work the point group symmetry and position of Eu in the MgAl2O4 crystal remain controversial and no effort was made at all to get such information which is very important to optimizing the optical output.
In our work Eu3+ will be doped in the MAS sample through combustion method. The research will be focussed on the spectroscopic properties of the Eu3+ ion, with emphasis on the local structure around Eu3+ ion, Judd–Ofelt analysis, and lifetime of the excited level and the exploration of the Eu3+ ion as a spectroscopic probe for site symmetry determination. We have used DFT based theoretical calculations to corroborate our PL data on local site occupancy and host–dopant energy transfer dynamics.
Such combined experimental and theoretical approach to understand the local site occupancy of europium ion and MAS/Eu3+ energy transfer dynamics has never been studied in lanthanide doped spinel material.
2. Experimental
2.1. Synthesis
MAS:Eu3+ has been synthesized using the method of gelation followed by citric acid assisted combustion. The raw materials used in this synthesis are citric acid (C6H8O7·H2O) (99.7%, AR grade), magnesium carbonate [Mg(CO3)2] (99%, AR grade) and aluminium nitrate [Al(NO3)3·9H2O] (98%, AR grade). The Mg2+ to citric acid molar ratio was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. At first magnesium carbonate and europium oxide was converted into nitrate form by dissolving in appropriate amount of nitric acid.
10. At first magnesium carbonate and europium oxide was converted into nitrate form by dissolving in appropriate amount of nitric acid.
Secondly, magnesium and aluminium nitrates were dissolved in minimum amount of quartz double distilled (QDD) water and then were mixed with europium nitrate solution. Citric acid was subsequently added to the resulting solution mixture.
The entire mixing process was done at room temperature under vigorous stirring at magnetic stirrer and kept as such for 1 hour. The solution mixture was then heated at 80 °C with continuous stirring for 5 hours, until a highly viscous gel is formed. The gel was heated under infra red lamp for 10–12 hours after which a brown ash-like voluminous product was formed. The ash like mass was then grounded and kept for calcination at 800 °C in a muffle furnace under air atmosphere for 12 h, after which a fine white powder was obtained.
2.2. Instrumental technique
Powders XRD patterns of the undoped and doped compounds were recorded using RIGAKU Miniflex-600 diffractometer operating in the Bragg–Brentano focusing geometry using Cu-Kα radiation (λ = 1.5406 Å) as an X-ray source with operating condition of 40 kV voltage and 30 mA current. The XRD patterns were taken in the 2θ range of 10° to 80° with scan rate of 1° per minute. Time resolved PL measurements were carried out on an Edinburgh CD-920 unit equipped with M300 grating monochromators (placed on either side of sample). The data acquisition and analysis were done by F900 software. A 150 W xenon flash lamp having variable frequency range of 10 to 100 Hz was used as the excitation source. Multiple emission and excitation scans (at least five) were taken to minimize the fluctuations in peak intensity and maximize signal–noise ratio. Approximately 25 mg of powder sample mixed with few drops of 4% collodion solution in the form of slurry was pasted over a glass plate. This was dried under ambient temperature and used for further studies.2.3. Computational methodology
The MgAl2O4 face-centered cubic spinel normal and inverse phases are studied using the Vienna ab initio simulation package (VASP),32,33 which calculate the Kohn–Sham eigen values within the framework of DFT. The calculations have been performed with the use of the generalized gradient approximation (GGA) and the exchange and correlation energy per electron have been described by the Perdew–Burke–Ernzerhof (PBE) parameterization.34 The interaction between electrons and atoms are described by means of the projector augmented-wave (PAW) method35 using Mg (3s – 2 valence electrons), Al (3s, 3p – 3 valence electrons), O (2s, 2p – 6 valence electrons) and Eu (5p, 6s, 5d – 9 valence electrons) as implemented in the VASP package. For normal cubic spinel unit-cell as well as structures comprises of oxygen vacancy (neutral and charged), optimization was carried out with respect to Ecut and k-point meshes to ensure convergence of total energy to within a precision 0.1 meV per atom. A Monkhorst–Pack36 k-space sampling of 13 × 13 × 13 for normal spinel and 7 × 7 × 7 for inverse spinel in reciprocal space for the Brillouin zone integration and a cutoff energy (Ecut) of 500 eV for the plane wave basis set was used. The total energy of normal and inverse cubic spinel unit-cell as well as structures comprises of oxygen vacancy (neutral and charged) were optimized with respect to volume (or lattice parameter) and atomic positions. Conjugate gradient algorithm was used for the unit-cell relaxations until the residual forces and stress in the equilibrium geometry were of the order of 0.005 eV Å−1 and 0.01 GPa, respectively. The final calculation of total electronic energy and density of states (DOS) were performed using the tetrahedron method with Blöchl corrections.373. Results and discussion
3.1. XRD study
Fig. 1 shows the XRD patterns of as-prepared sample 1.0 mol% Eu3+ doped MAS along with undoped MAS. XRD patterns of samples are in agreement with cubic system of MgAl2O4 (JCPDS no. 77-0435) which reveals that all the products are pure MgAl2O4 with Fd3m space group. The XRD patterns do not show any signature of pure oxide phase such as MgO, Al2O3 or Eu2O3 phase. The fact that such impurity phases are absent is an indication of homogeneous solid solution of MgAl2O4 and Eu3+, which further confirms the occupancy of Eu3+ ions in the lattice sites of Mg2+/Al3+ in MAS. It was also seen from the pattern that all peaks are sharp and well defined, indicating a good degree of crystallization or long range ordering. The synthesis temperature is 800 °C for both pure and europium doped sample and on comparison to reported literature our MAS samples mostly stabilizes in normal spinel form with octahedral Al3+ and tetrahedral Mg2+ sites.38 | ||
| Fig. 1 X-ray diffraction patterns of pure and Eu3+ doped MAS along with standard pattern corresponding to ICDD file no. 770435. | ||
3.2. SEM measurements
SEM micrograph depicted in Fig. S1 (ESI†) shows that most of particles are of 1–2 micron size. Large cluster could also be seen due to thermal induced agglomeration. There are large numbers of nanograins which are distributed on the facets of larger size particles. Interestingly some fluffy mass could also be seen which is a typical of reaction which leads to evolution of gaseous products such as CO, NOX. The particles have sharp faces and edges and looks like triangular plate.3.3. Luminescence study
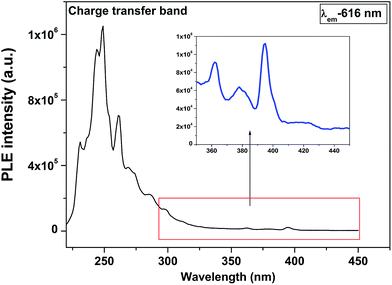
Fig. 3 shows the emission spectrum of undoped MAS under excitation wavelength of 230 nm. There are two different emission centre approximately around 440 nm and 510 nm. Such kind of multicolor emission is typical of a material relaxation takes place through several pathways and there exists various states within the band gap of materials.39 Excitation wavelength used in this case is 230 nm (∼5.39 eV) which is quiet less than the experimental band gap of MAS (∼7.8–9 eV);40 indicating the presence of certain localized energy states within the band gap of the material because transition directly from valence to conduction band will be forbidden in this case. This type of emission in inorganic powder can be because of local defects which arise during heating treatment of the sample. It is reported that MAS consists of various intrinsic defects, such as Mg2+, and O2− vacancies which normally exists as pair, known as Schottky defects.41 The emission peak around 440 nm in blue region is normally attributed to Mg2+ vacancies42–44 and the one at 510 nm in green region is attributed to oxygen vacancy.41
Fig. 4 shows the emission spectrum of 1.0 mol% Eu3+ doped MgAl2O4 under excitation with 256 nm corresponding to charge transfer state. The spectrum consists of various sharp lines due to the direct excitation of the Eu3+ ions from the ground level to higher levels of the 4f-manifold. There are five main features at 579, 592, 616, 654 and 704 corresponding to 5D0 → 7FJ (J = 0, 1, 2, 3 and 4). The main peaks at 592 and 616 nm corresponds to magnetic dipole transition (MDT) and hypersensitive electric dipole transition (EDT), respectively. The peak at 5D0 → 7F0 which is forbidden both by electric dipole as well as magnetic dipole transition could be seen is an indication of the fact that point group symmetry around Eu3+ in MAS is very low. It is reported that the 5D0 → 7F0 transition allowed in cases where following symmetries exists: Cs, C1, C2, C3, C4, C6, C2v, C3v, C4v, and C6v governed by electric dipole selection rule.45 The fact that 5D0 → 7F2 line at 616 nm (EDT) is intensed compared to 5D0 → 7F1 line at 592 nm (MD) indicates that local environment around Eu3+ is asymmetric without centre of inversion.
Eu3+ ions normally have high coordination numbers (>6) whereas the coordination numbers of Mg2+ and Al3+ ions in the MgAl2O4 normal spinel structure are just 4 and 6, respectively. Also, it is difficult for trivalent europium ion to enter the spinel lattice due to differences between their ion radii. Eu3+ ions (0.95 Å) in all probability substitutes in majority Mg2+ site (0.66 Å) in the crystal lattice because of the smaller differences between their ionic radii compared with Al3+ ions (0.51 Å). For each Eu3+ occupying Mg2+ sites there will be creation of positively charged defect [Eu3+]Mg2+. Also tetrahedrally coordinated Mg2+ ions lacks inversion centre which is further supporting our results. The fact that MDT is also observed in the emission spectrum could be because of the fact that some of the Eu3+ is also present at Al3+ site where there is no need for charge compensation.
Presence of defect induced broad host emission (same relative intensity as in pure form) in emission spectrum of europium doped MAS sample indicates negligible energy transfer at 1.0 mol% of dopant ion concentration. Similar observation was also seen in the work by Chen et al.24 where host emission could also be seen along with europium spectral features but they could not find out the reason behind it and left the question unattended. This we have tried to explain using DFT calculation in Section 3.3.4.
 | ||
| Fig. 5 Stark splitting pattern of 5D0–7F0, magnetic (ΔJ = ±1) and hypersensitive electric dipole transition (ΔJ = ±2) of Eu3+ in MAS. | ||
The substitutions of Mg2+/Al3+ with Eu3+ may results in substantial lattice distortion because of different ionic size and charges. From stark splitting pattern shown in Fig. 5, one, three and four peaks for 5D0–7F0, 5D0–7F1 (ΔJ = ±1) and 5D0–7F2 (hypersensitive, ΔJ = ±2) transition of Eu3+ were resolved for MAS:Eu3+ (1.0 mol%). According to the branching rules of various point groups,46 it infers that the actual site symmetry of Eu3+ in MAS reduces from original Td/Oh for Mg2+/Al3+ to C2v.
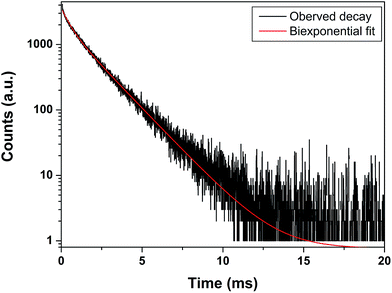
PL decay curve (Fig. 6) for MAS:Eu3+ was fitted to biexponential (n = 2) equation
 | (1) |
 | (2) |
Decay curves displaying two life-times (0.485 ms, 16% and 1.683 ms, 84%) indicate the presence of Eu3+ ions in two different chemical environments.
The percentage occupancy of Eu3+ ions exhibiting a specific life-time is obtained in such case using the formula
 | (3) |
Based on concept of phonon, a relatively longer luminescence lifetime should be attributed to a more symmetric site, as the f–f transition becomes more forbidden, whereas a shorter decay time is often associated with an asymmetric site due to relaxation in the selection rules. Species T2 (1.683 ms, 84%) arises because of Eu3+ ions occupying 4-coordinated Mg2+ site (MgO4) without inversion symmetry whereas species T1 (0.485 ms, 16%) can be attributed to Eu3+ ions occupying 6-coordinated Al3+ with inversion symmetry. These results also corroborate our emission studies where we have observed that Eu3+ ions occupy both Mg2+ as well as Al3+ sites but the fraction at Mg2+ site is almost 4 times more than at Al3+ site. The average lifetime is 1.49 ms.
MgAl2O4 spinel has a face-centered-cubic structure having space group Fd3m (O7h). Crystal structure of normal spinel is represented by lattice constant (a0) and oxygen parameter (u). In the normal spinel type, Al3+ and Mg2+ ions are in octahedral (local point group symmetry D3d) and tetrahedral coordination (local point group symmetry Td). Table 1 compares our GGA-PBE calculated a0, u, Mg–O and Al–O bond lengths with experimentally reported values and previous DFT calculated values using GGA and local density approximations (LDA). In this spinel type, our GGA-PBE calculated a0, u, Mg–O and Al–O bond lengths are 8.16 Å, 0.263, 1.96 and 1.94 Å, respectively, matching well with experimentally reported values (within 1%) and previously reported LDA and GGA study. GGA-PBE calculated equilibrium volumes (V0 in Å3) of defect structures  are 542.38, 535.42 and 529.79, respectively. Therefore, defect structural volumes are smaller compared to defect free structure.
are 542.38, 535.42 and 529.79, respectively. Therefore, defect structural volumes are smaller compared to defect free structure.
| Structural parameters | This study GGA-PBE | Previous experiments (neutron powder diffraction) | Previous DFT |
|---|---|---|---|
| Defect free | |||
| a0 (Å) | 8.160 | 8.084 (ref. 47) | 8.083 (LDA),48 8.107 (GGA-PW91)49 |
| V0 (Å3) | 543.34 | 0.2617 (ref. 47) | 0.2735 (LDA),48 0.2632 (GGA-PW91)49 |
| u | 0.2634 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Bond distances | |||
| Mg–O (Å) | 1.96 | — | 1.96 (LDA),48 1.96 (GGA-PW91)49 |
| Al–O (Å) | 1.94 | — | 1.82 (LDA),48 1.94 (GGA-PW91)49 |
In order to understand structural stability of site selective Eu doping in normal phase of MgAl2O4, the cohesive energies (E0) and equilibrium volumes (V0) were calculated after doping one Eu atom in Mg and Al site separately in the unit-cell of MgAl2O4 and full structural relaxations were performed as described in section Computational details. In our calculations we considered 1/56 (1.786%) doping level of Eu in normal phase of MgAl2O4 and results are shown in Table 2. Our DFT calculated cohesive energy difference of doped supercells (in Mg and Al positions separately) is 0.5846 eV for 56 atom unit-cell. Eu doping in Mg position is energetically more favorable than doping in Al position. Moreover, Eu doping in Mg and Al position increases the unit-cell volume by 18.36 and 17.51 Å3, compared to equilibrium volume of pure MgAl2O4 (543.34 Å3). Incorporation of Eu atom in the Mg site or Al site increases equilibrium volume of MgAl2O4.
| Position of Eu doping | ΔEcohesive in eV | Super-cell volume in Å3 |
|---|---|---|
| Mg position | 0 | 561.97 |
| Al position | 0.5846 | 560.85 |
In order to understand the change in electronic structure of normal spinel MgAl2O4 due to presence of the oxygen vacancy, we calculated DOS of  (neutral O vacancy),
(neutral O vacancy),  (+1 O vacancy) and
(+1 O vacancy) and  (+2 O vacancy) and those results are shown in ESI.† A detailed discussion on normal and inverse spinel MgAl2O4 can be found in ref. 54. In the defective unit-cell, Eu atom is doped in Mg site and the changes in the electronic DOS are shown in Fig. 7(b)–(d). Doping was done preferable on Mg site as it is energetically favorable compared to doping in Al site.
(+2 O vacancy) and those results are shown in ESI.† A detailed discussion on normal and inverse spinel MgAl2O4 can be found in ref. 54. In the defective unit-cell, Eu atom is doped in Mg site and the changes in the electronic DOS are shown in Fig. 7(b)–(d). Doping was done preferable on Mg site as it is energetically favorable compared to doping in Al site.
Fig. 7(b) shows the total and angular momentum decomposed DOS due to presence of neutral O vacancy. The spin-up and spin-down components are shown separately in upper and lower panels, respectively. Overall nature of the VB remains unaltered but an impurity band appears 2.7 eV ahead of VB maximum in the band-gap and below the Fermi level. This impurity band is mainly contributed by the Mg-s, p states. Moreover, an impurity bans also appears just below the CB, mainly contributed by Mg-s, p states. In this impurity band contribution from Eu d-states are negligible. But Eu d-states contribute strongly in the lower part of CB and bottom of the VB (above the impurity states).
Fig. 7(c) shows the total and angular momentum decomposed DOS due to presence of O vacancy with charge +2 ( ). Overall nature of the VB remains unaltered but an impurity band appears above VB maximum in the band-gap and just below the Fermi level. The impurity states are present 3.0 eV above the VB maximum. Impurity levels are composed of s, p states of Mg in the spin-up and spin-down components. Distribution of Eu d-states is very similar to the
). Overall nature of the VB remains unaltered but an impurity band appears above VB maximum in the band-gap and just below the Fermi level. The impurity states are present 3.0 eV above the VB maximum. Impurity levels are composed of s, p states of Mg in the spin-up and spin-down components. Distribution of Eu d-states is very similar to the  situation.
situation.
Fig. 7(d) shows the total and angular momentum decomposed DOS due to presence of O vacancy with charge +2 ( ). The spin-up and spin-down components are shown separately in upper and lower panels, respectively. Overall nature of the VB remains unaltered but two impurity bands appears above VB maximum in the band-gap. Impurity levels are composed of s, p states of Mg in the spin-up and spin-down components. Impurity states generated due to spin-up components are filled with electrons as it is situated just below the Fermi energy and impurity states generated due to spin-down components are empty. Impurities bands appear just below the CB minimum are similarly composed of s, p states of Mg. In this impurity band contribution from Eu d-states are negligible. But Eu d-states contribute strongly in the lower part of CB and bottom of the VB (above the impurity states). From this discussion it is evident that Eu states are solely localized in VB and CB regions and do not contribute in defects states. In our previous study we have shown that photo-luminescence properties of the MgAl2O4 are dominantly governed by the defect states coming from the presence of oxygen vacancies (neutral and charged). As a result photon energy transfer from host MgAl2O4 to dopant Eu is difficult.
). The spin-up and spin-down components are shown separately in upper and lower panels, respectively. Overall nature of the VB remains unaltered but two impurity bands appears above VB maximum in the band-gap. Impurity levels are composed of s, p states of Mg in the spin-up and spin-down components. Impurity states generated due to spin-up components are filled with electrons as it is situated just below the Fermi energy and impurity states generated due to spin-down components are empty. Impurities bands appear just below the CB minimum are similarly composed of s, p states of Mg. In this impurity band contribution from Eu d-states are negligible. But Eu d-states contribute strongly in the lower part of CB and bottom of the VB (above the impurity states). From this discussion it is evident that Eu states are solely localized in VB and CB regions and do not contribute in defects states. In our previous study we have shown that photo-luminescence properties of the MgAl2O4 are dominantly governed by the defect states coming from the presence of oxygen vacancies (neutral and charged). As a result photon energy transfer from host MgAl2O4 to dopant Eu is difficult.
Total and angular momentum decomposed density of states (DOS) of normal spinel pure MgAl2O4 and neutral oxygen vacancy in MgAl2O4 is depicted in Fig. S2 (ESI†). On the other hand total and angular momentum decomposed density of states (DOS) of normal spinel MgAl2O4 with +1 charged O defect ( ) and +2 charged O defect (
) and +2 charged O defect ( ) are shown in Fig. S3 (ESI†).
) are shown in Fig. S3 (ESI†).
3.4. Judd–Ofelt parameters and radiative properties
The Judd–Ofelt (J–O) theory has been successfully applied for the quantitative estimation of the photophysical properties of various trivalent rare earth ions. The Judd–Ofelt intensity parameters (Ω2,4) are the most important among all which provide valuable information about the local surrounding, degree of polarizability and covalency of rare earth ions. The details of all the calculations used here are explained extensively elsewhere.55It indeed is a very powerful tool for evaluating photophysical properties of europium ion in doped sample using the corrected emission spectrum. This is because of the fact that Eu3+ has pure magnetic dipole transition. For all other lanthanide ion; absorption spectrum is required. For the calculation corrected spectra (w.r.t. source, monochromator and detector) corresponding to 256 nm excitation wavelength is used. For MAS we have adopted value for the index of refraction of 1.7162 for calculation. Emission quantum efficiency of the emitting 5D0 level is written as
 | (4) |
| Transition | ARed (s−1) | ARmd (s−1) | ΩJ (10−21 cm2) | βJ (%) | η (%) | τR (ms) | τNR (s) |
|---|---|---|---|---|---|---|---|
| 5D0 → 7F1 | 0 | 73.6 | — | 15.7 | 69.7 | 2.14 (AR = 468 s−1) | 4.92 (ANR = 203 s−1) |
| 5D0 → 7F2 | 295 | 0 | 6.42 | 63.0 | |||
| 5D0 → 7F4 | 84.1 | 0 | 3.03 | 18.1 |
It is well known that the parameter Ω2, is an indication of the dominant covalent nature and/or structural changes in the vicinity of the Eu3+ ion (short range effects), while Ω4 intensity parameters are long range parameters that can be related to the bulk properties such as viscosity and rigidity of the inorganic matrices. The Ω2 parameter is related to the degree of covalence and polarizability of the chemical environment experienced by the Eu3+ ion; higher Ω2 values point to more covalent and polarizable environments. In case of MAS:Eu; Ω2 value was found to be greater than Ω4 indicating high covalency and low symmetry around europium ion which is also observed in emission spectrum wherein 5D0 → 7F2 (EDT) dominate 5D0 → 7F1 (MDT). This is well in agreement with extremely high asymmetry value (EDT/MDT ∼ 4.00) of Eu3+ in MAS. The calculated radiative transition rate (AR) for the excited 5D0 level of Eu3+ ion is found to be 468 s−1, which is higher than non-radiative transition rate (ANR = 203.2 s−1). The ANR can be attributed to nonradiative decays through different channels. This is an extremely encouraging result for making highly efficient red emitting phosphor material for white LEDs. The trend in branching ratio-suggests most of radiative energy (63%) goes in the 5D0 → 7F2 transition and least (15.7%) to 5D0 → 7F1. This is also observed from emission spectrum where 5D0 → 7F2 transition is much more intense than 5D0–7F1. The quantum efficiency of this particular phosphor which is defined as the ratio of the experimental lifetime to the calculated radiative lifetime of the 5D0 level is relatively high around 69.7%. High purity of red emission, high radiative transition rate coupled with good fluorescence quantum yields highlight the potential of MAS:Eu3+ as a promising red phosphor.
To evaluate the material performance for phosphor application, CIE chromaticity coordinates were evaluated for MAS:Eu3+ (1.0 mol%) sample adopting standard procedures. This is represented as the point ‘*’ in the CIE diagram shown in Fig. 8. It is clear from the values that, MAS:Eu red emission with very high quantum efficiency of 69.7%. This is because of the fact that non-radiative losses compared to radiative emission is very less in this particular system.
4. Conclusion
Undoped and 1.0 mol Eu3+ doped magnesium aluminate spinel (MAS) was synthesized using citric acid assisted combustion technique. Their phase purity and optical characteristics were analyzed by powder X-ray diffraction (XRD) and time resolved photoluminescence spectroscopy (TRPLS). Theoretical calculations were performed on both the sample Vienna ab initio simulation package (VASP). Emission profile of undoped MAS sample display blue and green band on UV irradiation which were respectively attributed to magnesium and oxygen vacancy. On doping europium two phenomenons were observed (i) higher fraction of europium ion is localized at Mg2+ site and (ii) absence of host–dopant energy transfer process. Lifetime spectroscopy reveals that Eu3+ ions occupy both Mg2+ as well as Al3+ sites but the fraction at Mg2+ site is almost 4 times more than at Al3+ site. DFT calculations also showed Eu3+ doping in Mg2+ position is energetically more favorable than doping at Al3+ site. Emission spectrum of doped sample consists of host as well as Eu3+ emission indicating the absence of complete host to dopant energy transfer. Our previous work on MAS54 shows that emission in MAS is due to defect states mainly from oxygen vacancies (neutral and charged). Theoretical measurements of Europium doped MAS sample reveals that Eu3+ states are solely localized in valence and conduction band regions and do not contribute in defects states. As a result photon energy transfer from host MAS to dopant Eu is difficult. Based on the stark splitting pattern in emission profile of Eu3+ doped MAS the actual site symmetry for europium ion was also evaluated which comes out to be C2v. Judd–Ofelt analysis reflects higher Ω2 value compared to Ω4; indicating high covalency and low symmetry around europium ion which is also observed in emission spectrum. High purity of red emission coupled with good fluorescence quantum yields highlights the potential of this unexplored MAS as a promising phosphor.References
- (a) J.-C. G. Bunzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC; (b) A. Arakcheeva, D. Logvinovich, G. Chapuis, V. Morozov, S. V. Eliseeva, J. C. G. Bunzli and P. Pattison, Chem. Sci., 2012, 3, 384 RSC; (c) P. A. Tanner, Chem. Soc. Rev., 2013, 42, 5090 RSC.
- (a) B. R. Judd, Phys. Rev., 1962, 127, 750 CrossRef CAS; (b) G. S. Ofelt, J. Chem. Phys., 1962, 37, 511 CrossRef CAS; (c) S. K. Gupta, P. S. Ghosh, M. Sahu, K. Bhattacharyya, R. Tewari and V. Natarajan, RSC Adv., 2015, 5, 58832 RSC.
- W. Zheng, P. Huang, D. Tu, E. Ma, H. Zhu and X. Chen, Chem. Rev., 2015, 44, 1379 RSC . Refernce therein.
- (a) R. Shukla, S. K. Gupta, V. Grover, V. Natarajan and A. K. Tyagi, Dalton Trans., 2015, 44, 10628 RSC; (b) S. K. Gupta, K. Sudarshan, P. S. Ghosh, K. Sanyal, A. P. Srivastava, A. Arya, P. K. Pujari and R. M. Kadam, RSC Adv., 2016, 6, 3792 RSC.
- S. K. Gupta, B. Rajeshwari, S. N. Achary, S. J. Patwe, A. K. Tyagi, V. Natarajan and R. M. Kadam, Eur. J. Inorg. Chem., 2015, 2015, 4429 CrossRef CAS.
- S. K. Gupta, M. Sahu, P. S. Ghosh, D. Tyagi, M. K. Saxena and R. Kadam, Dalton Trans., 2015, 44, 18957 RSC.
- R. Phatak, S. K. Gupta, K. Krishnan, S. K. Sali, S. V. Godbole and A. Das, Dalton Trans., 2014, 43, 3306 RSC.
- L. K. Bharat, Y. I. I. Jeon and J. S. Yu, J. Alloys Compd., 2014, 614, 443 CrossRef.
- L. K. Bharat and J. S. Yu, J. Nanosci. Nanotechnol., 2013, 13, 8239 CrossRef CAS PubMed.
- P. A. Primus, A. Menski, M. P. Yeste, M. A. Cauqui and M. U. Kumke, J. Phys. Chem. C, 2015, 119, 10682 CAS.
- V. I. Parvulescu and C. Tiseanu, Catal. Today, 2015, 253, 33 CrossRef CAS.
- S. K. Gupta, P. S. Ghosh, K. Sudarshan, R. Gupta, P. K. Pujari and R. M. Kadam, Dalton Trans., 2015, 44, 19097 RSC.
- M. P. Pico, I. Álvarez-Serrano, M. L. López and M. L. Veiga, Dalton Trans., 2014, 43, 14787 RSC.
- B. K. Chatterjee, K. Bhattacharjee, A. Dey, C. K. Ghosh and K. K. Chattopadhyay, Dalton Trans., 2014, 43, 7930 RSC.
- H. Y. Wang, S. F. Hung, H. Y. Chen, T. S. Chan, H. M. Chen and B. Liu, J. Am. Chem. Soc., 2016, 138, 36 CrossRef CAS PubMed.
- T. Nakajima and T. Tsuchiya, J. Mater. Chem. C, 2015, 3, 3809 RSC.
- S. Patra, E. Roy, P. Karfa, S. Kumar, R. Madhuri and P. K. Sharma, ACS Appl. Mater. Interfaces, 2015, 7, 9235 CAS.
- I. Ganesh, Int. Mater. Rev., 2013, 58, 63 CrossRef CAS.
- Y. Kim, J. Lim and S. Kang, J. Mater. Chem. C, 2015, 3, 8970 RSC.
- (a) R. J. Wiglusz, G. Boulon, Y. Guyot, M. Guzik, D. Hreniak and W. Strek, Dalton Trans., 2014, 43, 7752 RSC; (b) G. Boulon, G. Alombert-Goget, Y. Guyot, M. Guzik, T. Epicier, N. P. Blanchard, L. Chen, L. Hu and W. Chen, J. Mater. Chem. C, 2014, 2, 9385 RSC.
- (a) D. Mei, V. L. Dagle, R. Xing, K. O. Albrecht and R. A. Dagle, ACS Catal., 2016, 6, 315 CrossRef CAS; (b) J. Kehres, J. W. Andreasen, J. B. Fløystad, H. Liu, A. Molenbroek, J. G. Jakobsen, I. Chorkendorff, J. H. Nielsen, K. Høydalsvik, D. W. Breib and T. Vegge, J. Phys. Chem. C, 2015, 119, 1424 CrossRef CAS.
- R. Zhang, M. Liu, L. Lu, S. Mi and H. Wang, J. Mater. Chem. C, 2015, 3, 5598 RSC.
- K. E. Sickafus, J. M. Wills and N. W. Grimes, J. Am. Ceram. Soc., 1999, 82, 3279 CrossRef CAS.
- X. Y. Chen, C. Ma, Z. J. Zhang and X. X. Li, Microporous Mesoporous Mater., 2009, 123, 202 CrossRef CAS.
- X. Y. Chen, C. Ma and S. P. Bao, Solid State Sci., 2010, 12, 857 CrossRef CAS.
- I. Omkaram, B. V. Rao and S. Buddhudu, J. Alloys Compd., 2009, 474, 565 CrossRef CAS.
- R. J. Wiglusz, T. Grzyb, S. Lis and W. Strek, J. Lumin., 2010, 130, 434 CrossRef CAS.
- W. Guan, J. Li and X. Wang, Phys. Status Solidi A, 2014, 211, 1778 CrossRef CAS.
- I. E. Kolesnikov, E. V. Golyeva, A. V. Kurochkin and M. D. Mikhailov, J. Alloys Compd., 2016, 654, 32 CrossRef CAS.
- S. J. Yoon, D. A. Hakeem and K. Park, Ceram. Int., 2016, 42, 1261 CrossRef CAS.
- I. V. Beketov, A. I. Medvedev, O. M. Samatov, A. V. Spirina and K. I. Shabanova, J. Alloys Compd., 2014, 586, S472–S475 CrossRef CAS.
- G. Kresse and J. Furthmueller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 5, 11169 CrossRef.
- G. Kresse and J. Furthmueller, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3685 CrossRef PubMed.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 13, 5188 CrossRef.
- P. E. Blochl, O. Jepsen and O. K. Andesen, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 4, 16223 CrossRef.
- R. J. Wiglusz, G. Boulon, Y. Guyot, M. Guzik, D. Hreniak and W. Strek, Dalton Trans., 2014, 43, 7752 RSC.
- V. M. Longo, L. S. Cavalcante, A. T. De Figueiredo, L. P. S. Santos, E. Longo, J. A. Varela, J. R. Sambrano and A. C. Hernandes, Appl. Phys. Lett., 2007, 90, 091906 CrossRef.
- A. V. Emeline, G. V. Kataeva, V. K. Ryabchuk and N. Serpone, J. Phys. Chem. B, 1999, 103, 9190 CrossRef CAS.
- J. H. Lim, B. N. Kim, Y. Kim, S. Kang, R. J. Xie, I. S. Chong, K. Morita, H. Yoshida and K. Hiraga, Appl. Phys. Lett., 2013, 102, 031104 CrossRef.
- Y. Fujimoto, H. Tanno, K. Izumi, S. Yoshida, S. Miyazaki, M. Shirai, K. Tanaka, Y. Kawabe and E. Hanamura, J. Lumin., 2008, 128, 282 CrossRef CAS.
- T. Sato, M. Shirai, K. Tanaka, Y. Kawabe and E. Hanamura, J. Lumin., 2005, 114, 155 CrossRef CAS.
- A. Jouini, A. Yoshikawa, A. Brenier, T. Fukuda and G. Boulon, Phys. Status Solidi C, 2007, 4, 1380 CrossRef CAS.
- X. Y. Chen and G. K. J. Liu, J. Solid State Chem., 2005, 178, 419 CrossRef CAS.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1 CrossRef CAS.
- R. C. Peterson, G. A. Lager and R. L. Hitterman, Am. Mineral., 1991, 76, 1455 CAS.
- S. Wei and S. B. Zhang, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 045112 CrossRef.
- S. M. Hosseini, Phys. Status Solidi B, 2008, 245, 2800 CrossRef CAS.
- (a) M. L. Bortz, R. H. French, D. J. Jones, R. V. Kasowski and F. S. Ohuchi, Phys. Scr., 1990, 41, 4404 CrossRef; (b) M. L. Boltz and R. H. French, Appl. Phys. Lett., 1989, 55, 1955 CrossRef.
- S. K. Gupta, P. S. Ghosh, N. Pathak, A. Arya and V. Natarajan, RSC Adv., 2014, 4, 29202 RSC.
- S. K. Gupta, P. S. Ghosh, A. Arya and V. Natarajan, RSC Adv., 2014, 4, 51244 RSC.
- N. Pathak, S. K. Gupta, P. S. Ghosh, A. Arya, V. Natarajan and R. M. Kadam, RSC Adv., 2015, 5, 17501 RSC.
- N. Pathak, P. S. Ghosh, S. K. Gupta, S. Mukherjee and R. M. Kadam, J. Phys. Chem. C, 2016, 120, 4016 CAS.
- S. K. Gupta, M. Mohapatra, S. V. Godbole and V. Natarajan, RSC Adv., 2013, 3, 20046 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03369e |
| This journal is © The Royal Society of Chemistry 2016 |