Bacterial protease-triggered clove oil release from proteoliposomes against S. aureus biofilms on dried soybean curd
Abstract
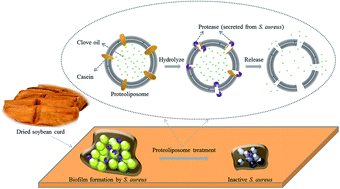
The purpose of this study is to develop a new approach to deliver antimicrobials against bacterial infections by taking advantage of the hydrolysis of protease by casein. Clove oil was encapsulated into proteoliposomes (liposomes inlaid with casein) to enhance their stability and prolong action time. The controlled release of clove oil from proteoliposomes was triggered via bacterial proteases secreted from S. aureus. The average particle size of proteoliposomes containing clove oil was 701.5 nm with a polydispersity index (PDI) of 0.019, zeta potential of −38.6 mV and encapsulation efficiency of clove oil of 40.01%. The antibiofilm activities of proteoliposomes containing clove oil against S. aureus on dried soybean curd were assessed by a colony forming units (CFU) counting method. Compared to traditional liposomes, the application of the proteoliposomes could further enhance the antibiofilm effect of an essential oil against food-borne pathogens.

 Please wait while we load your content...
Please wait while we load your content...