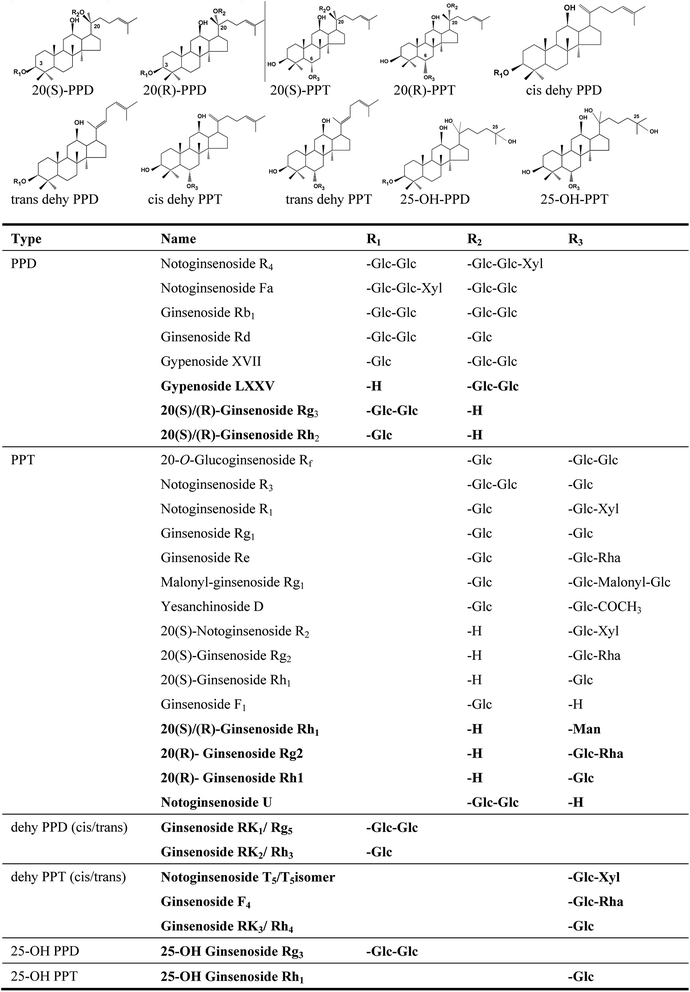
Structure-based prediction of CAD response factors of dammarane-type tetracyclic triterpenoid saponins and its application to the analysis of saponin contents in raw and processed Panax notoginseng†
Ming Pengab,
Tong Zhang*c,
Yue Dingc,
Yaxiong Yia,
Yongjian Yangb and
Jian Leb
aSchool of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
bDepartment of Chemistry, Shanghai Institute for Food and Drug Control, Shanghai, 201203, China
cExperiment Center for Teaching and Learning, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China. E-mail: zhangtdmj@hotmail.com; Fax: +86 21 51322337; Tel: +86 21 51322318
First published on 1st April 2016
Abstract
The analysis of saponin contents in Panax notoginseng (Sanqi) is challenged by the lack of authentic reference standards. In this study, a gradient eluted HPLC method coupled with charged aerosol detector (CAD) has been established to solve this problem. The impact of structural features, including the type of aglycon, the optical rotations at C-20, the glycosyl substituent and the glycosyl linkage of dammarane-type tetracyclic triterpenoid saponins on their CAD response factors has been discovered. The rules of the impact have been utilized to predict CAD response factors of saponins in raw and processed notoginseng based on their structures elucidated by LC-QTOFMS. An intensive investigation of the saponin contents in raw (different cultivate places, sizes, and medicinal parts) and processed (steaming, baking, autoclaving, stewing and frying) Panax notoginseng were implemented. This method was successfully applied to distinguishing the quality of raw and processed Panax notoginseng, finding out biomarkers in processed notoginseng, and screening the best processing technique for this herb.
1. Introduction
Notoginseng, the dry root or rhizome of Panax notoginseng (Burk.) F. H. Chen (P. notoginseng), also called ‘Sanqi’ or ‘Sanchi’, is a precious traditional Chinese medicine with a long history of medical use. The saponin components have been discovered to contribute to the main pharmacological functions of this herb, such as the treatment of cardiovascular diseases,1 the biological activities of anti-cancer,2 anti-hyperlipoidemia,3 and anti-hyperglycemia,4 etc. In traditional Chinese medical applications, processed notoginseng is distinguished from the raw herb by the claim of its capability to “nourish” blood.5 Furthermore, contemporary researches have reported that processed notoginseng exhibit more potent pharmaceutical activities than raw notoginseng, such as anticancer,6–8 antiplatelet, anticoagulant, and platelet aggregation inhibition effects,9 etc. Apparently, different compound basis of raw and processed notoginseng directly influences their pharmacological activities.Dammarane-type tetracyclic triterpenoid saponins have been found to be the major active components in P. notoginseng,10 and can essentially be classified into two types: protopanaxadiol (PPD) and protopanaxatriol (PPT) type. The lacking of authentic reference standards of rare saponins, especially those secondary saponins only existed in processed notoginsengs has impeded the quality control of this herb. Recently, some strategies of quantitative analysis of multi-component with single marker (QAMS) have been developed for the determination of saponin content in P. notoginseng mostly based on HPLC-UV and LC-MS platforms. A QAMS method focused on 11 saponins in P. notoginseng has been established and validated at UV 200 nm.11 The slopes of the equations of linear regressions for each saponin were used to calculate the relative correction factor (RCF). Although this method is simple and accurate, the RCFs of each saponin need to be calculated before the testing on real samples. Moreover, the RCFs of those saponins without authentic reference available are still not achievable and predicted, and the intensive analysis of the whole saponin contents in notoginsengs, especially those in processed herbs could not easily be accomplished. Lai et al.12 developed a green protocol for the utilizing of specific enzymatic hydrolyzing process to calculate relative response factor of specific PPD type saponins, with less consumption of solvent and authentic reference standards. However, this protocol has only focused on 4 PPD saponins so far. Further researches are needed to find the specific enzymes for the hydrolyzing of other types of saponins. Moreover, a HPLC-ESI-MS coupled with mobile-phase compensation method has been investigated for the determination of saponins in P. notoginseng calculated based on normalized data of saponin peaks.13 However, the variations of MS responses of different saponins owing to their structural types and molecular weights could still not be neglected, which limits the extensive application of this method.
Charged aerosol detector (CAD) was firstly introduced in 2002.14 CAD is a mass sensitive and universal detector for the routine determination of any non-volatile and many semi-volatile chemical species. The liquid mobile phase is nebulized in CAD chamber by N2 to become aerosol droplets. Then the small droplets containing analytes enter the drying tube, and the big droplets which are composed of the majority of mobile phase enter the wasting tube. After that, the dry particles are mixed with a charged N2 gas flow which has just passed through the corona discharge needle, and at the meantime the charges are transferred to the dry particles. The charged analyte particles are then collected and the electrical charges are measured with an electrometer. CAD has extensively been applied for the analysis of impurities in pharmaceuticals,15,16 food products and herbal dietary supplements,17–19 pharmaceutical formulations,20,21 and environmental pollutants,22etc. Moreover, HPLC-CAD has been performed on the analysis of major saponins in raw notoginseng by external standard method using commercially available reference standards.23,24 However, the content of those minor saponins were not mentioned due to the absence of authentic reference standards. CAD was claimed to be generating identical peak response for all non-volatile substances, however, quite a few studies have also investigated that CAD responses of the analytes are not always the same.25–27 The variations of the responses may be due to the particle density, hygroscopicity, and volatility, etc., of the analytes in the particle phase during nebulization.28 This means that it is inappropriate to arbitrarily assume an identical CAD response for all the saponins which embrace close but different structures without figuring out the their relationships. However, once the relation between the saponin structure and its CAD response is elucidated, this detector is still a convenient and stable detector for the determination of saponins which are short of chromophores in their structure.
In this article, a gradient eluted HPLC-CAD method with post-column mobile phase compensation has been developed to determine the saponin contents in raw and processed P. notoginseng. The impact of the structural features, including types of aglycon, optical rotation, glycosyl substituent and glycosyl linkage of dammarane-type tetracyclic triterpenoid saponins on their CAD response factors (RFs) has been discovered. Moreover, the rules have been successfully utilized to predict CAD RFs of the saponins based on their structures, which were identified by LC-QTOFMS in our study, and the prediction has also been validated. An in-depth investigation on saponin contents in raw P. notoginseng of different sizes, cultivated places and medicinal parts, as well as the secondary saponins and biomarkers in processed notoginseng of different processing procedures was then implemented.
2. Experimental
2.1. Chemicals and reagents
Reference standards of notoginsenoside R1, ginsenoside Rg1, Re, Rf, Rb1, 20(S)-Rg2, 20(S)-Rh1, 20(R)-Rg2, 20(R)-Rh1, Rb2, Rb3, F1, Rd, F2, 20(S)-Rg3, 20(R)-Rg3, 20(S)-PPT, CK, 20(R)-Rh2 and 20(R)-PPD were purchased from Chengdu Must Bio-technology Co. LTD. Ginsenoside 20(S)-Rh2 and 20(S)-PPD were kindly supplied by Shanghai Pharm Valley Co. LTD. Except for 20(R)-Rh1 (purity 97.65%), 20(R)-Rh2 (purity 92.33%) and 20(S)-PPD (purity 95.99%), the purities of all the above reference standards were labeled above 98% by the manufacturers. Reference standard of gypenoside XVII was obtained from Shanghai Winherb Medical Science Co., Ltd (purity 98.93%). Tinidazole (purity 100%) was provided by Zhejiang Supor Pharmaceuticals Co., Ltd. HPLC grade acetonitrile and methanol were obtained from Merck (Darmstadt, Germany). Deionized water was purified using a Milli-Q system (Millipore, Bedford, MA).2.2. Raw herb of Panax notoginseng
Main roots of raw P. notoginseng were purchased from two cultivated places in China. Seven different sizes of main root (20, 30, 40, 60, 80, 120 and countless heads) were purchased from Wenshan county, Yunnan province. And five different sizes of main root (30, 40, 60, 120 and countless heads) were purchased from Bobai county, Guangxi province. The term “head” in Panax notoginseng refers to the size of this herb, which has been used in China for a long history. It refers to the number of pieces of notoginseng main roots per 0.5 kg. For example, “40 heads” in this article means that each 0.5 kg of notoginseng herb contains 40 pieces. Apparently, the greater the number of head is, the smaller is the size of main root. In Chinese market, the smaller the number of head is, the more expensive is the notoginseng, because people believe that notoginseng of bigger sizes contain greater amount of total saponins, thus have more potent pharmacology effects. In addition, other medicinal parts of P. notoginseng (rhizome, branch root and root hair) were all obtained from Wenshan county. All the raw P. notoginsengs were pulverized and screened through an 80 mesh sieve. Water contents of the notoginseng powders were determined by Karl Fischer method.2.3. Processing procedures of notoginseng
Processed notoginsengs were prepared by different procedures, i.e., steaming, autoclaving, baking, stewing and frying. All the processed notoginsengs were prepared using raw 120-head main root of P. notoginseng cultivated in Wenshan county. All the processed notoginsengs were dried under vacuum at 80 °C for 48 h before being pulverized. Then the pulverized powder was being screened through an 80 mesh sieve. Karl Fischer titration was performed afterwards to determine the water contents of all processed notoginseng powder.2.4. Preparation of reference standard stock solution and internal standard solution
The stock reference solution was prepared using 70% methanol aqueous solution. Since the contents of different saponins vary greatly, the concentrations of 22 reference standards in stock solution ranged from 0.05–1.5 mg mL−1 according to the saponin contents in notoginseng. Tinidazole was dissolved in 70% methanol aqueous solution to prepare an internal standard (IS) solution at the concentration of 2 mg mL−1.2.5. Preparation of sample solution
About 0.5 g of notoginseng powder was accurately weighed and transferred into a 20 mL volumetric flask. Then 5 mL of IS solution and 10 mL of 70% methanol aqueous solution was added into the volumetric flask. The flask was ultra-sonicated (500 W, 50 Hz) for 60 min, and 70% methanol aqueous solution was added to volume afterwards. After the sample solution was shaken well and standing for a while, the supernatant was withdrawn and filtered through a 0.22 μm polytetrafluoroethylene (PTEE) filter.2.6. HPLC chromatographic conditions
HPLC analysis was performed on a Dionex Ultimate 3000 series HPLC system, equipped with vacuum degasser, dual gradient pump, autosampler, ultraviolet detector, and Corona Ultra charged aerosol detector (Munich, Bavaria, Germany). A Waters HSS C18 column (25 cm × 4.6 mm, i.d., 3.5 μm, Ireland) was used for chromatographic separation at 30 °C. The separation was achieved using a binary gradient elution system consisted of water and acetonitrile (ACN) as mobile phases. The gradient program was as follows: 0–31 min (20.5% ACN), 31–32 min (20.5% → 30% ACN), 32–50.5 min (30% → 35% ACN), 50.5–61 min (35% → 50% ACN), 61–81 min (50% → 90% ACN), 81–91 min (90% ACN). The flow rate was set at 1.0 mL min−1 and the sample injection volume was 10 μL. The sample elution was eluted to UV and CAD detector successively. UV detector wavelength was set at 203 nm. CAD data collection frequency was 2 Hz, and nebulizer temperature was 35 °C.In order to keep the organic modifier content to be constant when the mobile phases reached CAD detector, post column compensation of mobile phases was introduced. Since dual gradient pumps of this HPLC system had a slight difference in the dead volume, the post column counter gradient program for CAD detector was set for a 0.3 min's delay, which was as follows: 0–31.3 min (79.5% ACN), 31.3–32.3 min (79.5% → 70% ACN), 32.3–50.8 min (70% → 65% ACN), 50.8–61.3 min (65% → 50% ACN), 61.3–81.3 min (50% → 10% ACN), 81.3–91.0 min (10% ACN), with the total flow rate of 1.0 mL min−1.
2.7. Validation of HPLC method
2.8. LC-MS conditions
LC-MS data were acquired on an Agilent 1290 Infinity UPLC coupled with Agilent 6538 UHD Accurate-Mass QTOF LC/MS system and an ESI source (Agilent Technologies, Santa Clara, USA). The chromatographic conditions, including type of chromatographic column, column temperature, gradient elution, flow rate and injection volume, were exactly the same as those of HPLC-CAD system, except that the mobile phase A was 0.01% formic acid instead of water. A post-column tee joint was used to split the flow rate, and the actual flow rate passed through ESI source kept 0.2 mL min−1 constantly. The optimized mass parameters were as follows: electrospray ion (ESI) source; gas temperature, 350 °C; drying gas (N2), 10 L min−1; nebulizing gas pressure, 40 psig; capillary voltage, 3500 V; capillary current, 0.032 μA; chamber current, 2.20 μA. ESI negative and positive modes were performed on both MS and tandem MS in the m/z range of 100–1400. Besides, MS/MS analysis was achieved using collision energies of 10 V, 20 V, 30 V and 40 V, respectively. Prior to mass data acquisition, the mass spectrometry was tuned and optimized using Agilent ESI-L low concentration Tunning mix (lot: LB95102). The accurate mass was measured to identify the structures of saponins in raw and processed notoginsengs using Agilent MassHunter Workstation software (Version B.04.00).2.9. Prediction of CAD response
In this article, 22 dammarane-type tetracyclic triterpenoid saponins or aglycons with authentic reference standards were utilized to set up the HPLC-CAD method. And these above 22 saponins have covered the scope of different structure features, including type of aglycon, optical rotation at C-20, glycosyl substituent and glycosyl linkage, of the saponins basically existed in notoginsengs. In this experiment, the slope of calibration curve for each known saponin was regarded as the RF of its chromatographic peak. According to our results, the differences of CAD RFs of saponins with different structures were much smaller than UV RFs. The impact of structural features of PPD and PPT saponins on the CAD RFs has been investigated. Since the structures of all the saponins in notoginsengs have already been identified by LC-MS, the CAD RFs of saponins without authentic reference standards could then be predicted.2.10. Validation of the prediction of CAD RFs
The prediction of CAD RFs was validated by some of the saponins with authentic reference standards. The differences between their CAD slopes obtained from the linear regressions and their predicted CAD RFs are calculated as: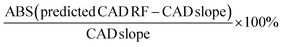 . Ten PPT type saponins with authentic reference standards in this article were regarded as sample saponins so that their CAD RFs were going to be assigned based on the rule of impact of their structural features. Furthermore, another authentic reference standard of PPD type saponin, namely gypenoside XVII, was chosen to perform the validation. The stock validation solution was prepared using 70% methanol aqueous solution. A series of validation solutions were prepared by diluting the stock validation solution for 6 concentration levels, and the concentrations were between 0.0041 and 0.1220 mg mL−1. In each level of validation solutions, IS maintained a constant concentration of 0.5 mg mL−1. The validation solutions were then injected into HPLC under the developed chromatographic condition. Then the CAD linear regression was set up by plotting the peak area ratio versus IS against the concentration. The difference between CAD slope of linear regression and the predicted RF value was calculated.
. Ten PPT type saponins with authentic reference standards in this article were regarded as sample saponins so that their CAD RFs were going to be assigned based on the rule of impact of their structural features. Furthermore, another authentic reference standard of PPD type saponin, namely gypenoside XVII, was chosen to perform the validation. The stock validation solution was prepared using 70% methanol aqueous solution. A series of validation solutions were prepared by diluting the stock validation solution for 6 concentration levels, and the concentrations were between 0.0041 and 0.1220 mg mL−1. In each level of validation solutions, IS maintained a constant concentration of 0.5 mg mL−1. The validation solutions were then injected into HPLC under the developed chromatographic condition. Then the CAD linear regression was set up by plotting the peak area ratio versus IS against the concentration. The difference between CAD slope of linear regression and the predicted RF value was calculated.
2.11. Saponin content determination of notoginseng
The saponin contents of real notoginseng samples were calculated using internal standard method based on the CAD peak area ratio versus IS for each analyte. For the saponins with authentic reference standard available, an internal standard curve method was applied based on their validated calibration curve equations. Nevertheless, for those saponins without reference standards available in this experiment, their CAD RFs were predicted based on the structures identified by LC-MS. Thus, the content of each saponin was calculated using a simple internal standard method according to the predicted CAD RFs.3. Results and discussion
3.1. Optimisation of gradient elution program
Based on the polarities, the 22 saponins with authentic reference standards available in this experiment can be divided into three groups: (1) high polarity saponins, including R1 and Rg1; (2) medium polarity saponins, including Re, Rf, Rb1, 20(S)-/20(R)-Rg2, 20(S)-/20(R)-Rh1, Rb2, Rb3, F1 and Rd; (3) low polarity saponins, including F2, 20(S)-/20(R)-Rg3, CK, 20(S)-/20(R)-Rh2, 20(S)-PPT and 20(S)-/20(R)-PPD. Since there is one more hydroxyl group in PPT aglycon than in PPD aglycon, the polarities of PPT type saponins are basically higher than those of PPD type saponins, given the same number and type of glycosyl substituents. The most difficult part of establishing this LC method is the separation of optical isomers, e.g., 20(S)-/20(R)-Rg3 and 20(S)-/20(R)-Rh2, and geometric isomers at C-20 position, e.g., RK3/Rh4, and RK1/Rg5, since these isomers bear very similar polarities and close chemical properties. Gradient elution program was set up, and the saponin peaks were successfully separated. In the sample chromatogram of raw notoginsengs, the retention times of all the saponins were within 60 min, which indicated that the saponins were of high and medium polarities. However, upon steaming for 3 h, low polar saponins emerged in processed notoginseng. Furthermore, the content of low polar saponins increased dramatically when notoginsengs were autoclaved at 120 °C for 18 h (Fig. 1).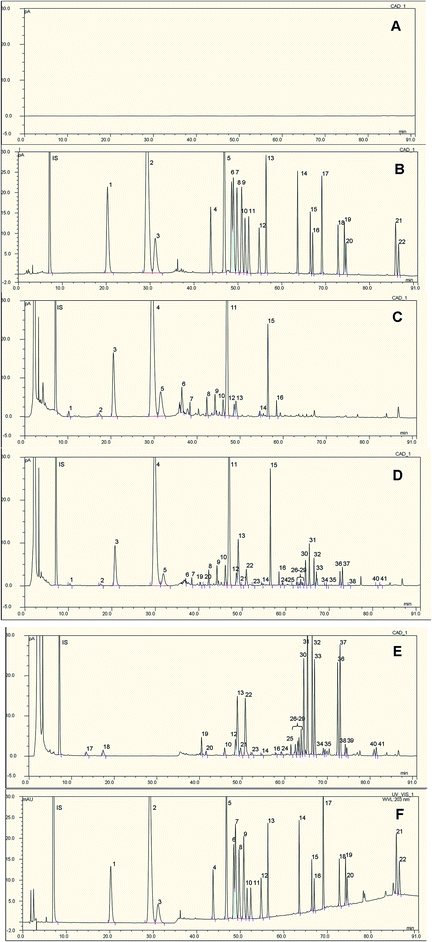 | ||
| Fig. 1 Typical HPLC-CAD chromatograms of blank (A), reference standard solution (B), sample solutions of raw notoginseng (40 head, Yunnan) (C), steamed notoginseng (100 °C, 3 h) (D), and autoclaved notoginseng (120 °C, 18 h) (E), and typical HPLC-UV chromatogram of reference standard solution (F). The peak numbers denoted in reference standard solution (B) and (F) are: 1, R1; 2, Rg1; 3, Re; 4, Rf; 5, Rb1; 6, 20(S)-Rg2; 7, 20(S)-Rh1; 8, 20(R)-Rg2; 9, 20(R)-Rh1; 10, Rb2; 11, Rb3; 12, F1; 13, Rd; 14, F2; 15, 20(S)-Rg3; 16, 20(R)-Rg3; 17, 20(S)-PPT; 18, compound K; 19, 20(S)-Rh2; 20, 20(R)-Rh2; 21, 20(S)-PPD; 22, 20(R)-PPD. The peak numbers denoted in sample solution (C), (D) and (E) are: 1, 20-O-glucoginsenoside Rf; 2, R3; 3, R1; 4, Rg1; 5, Re; 6, malonyl-ginsenoside Rg1; 7, yesanchinoside D; 8, R4; 9, Fa; 10, 20(S)-R2; 11, Rb1; 12, 20(S)-Rg2; 13, 20(S)-Rh1; 14, F1; 15, Rd; 16, gypenoside XVII; 17, 20(S)-25-OH Rh1; 18, 20(R)-25-OH Rh1; 19, 20(S)-Rh1 (Man as glycosyl substituent); 20, 20(R)-Rh1 (Man as glycosyl substituent); 21, 20(R)-Rg2; 22, 20(R)-Rh1; 23, 25-OH Rg3; 24, gypenoside LXXV; 25, gypenoside LXXV isomer; 26, T5; 27, U; 28, T5 isomer; 29, F4; 30, RK3; 31, Rh4; 32, 20(S)-Rg3; 33, 20(R)-Rg3; 34, unknown 1#; 35, unknown 2#; 36, RK1; 37, Rg5; 38, 20(S)-Rh2; 39, 20(R)-Rh2; 40, RK2; 41, Rh3. The peak numbers denoted in sample solutions are the same as in Tables 2 and 3. | ||
3.2. Optimisation of post column compensation program of CAD detector
One of the distinguishing features of CAD detector is that different compounds theoretically exhibit similar responses. However, the amount of organic modifier in the mobile phase significantly influences the CAD response. It has been reported that with the increasing of organic modifier from 0% to 100% in the mobile phase, the CAD response may increase dramatically from 5 to 10 times.29 Unfortunately, the wide range of polarities of saponins in raw and processed notoginsengs hinders the use of isocratic elution of mobile phase. Dual pump HPLC system has been invented to overcome this restraint. A counter gradient program was designed for post column compensation, and a constant quantity of organic modifier in mobile phase reached the CAD detector at any time. Due to a slight difference in the dead volumes of the dual pump system, a 0.3 minute time lag was considered in the post column counter gradient program.The total content of ACN in mobile phase in post-column gradient elution was evaluated. It has been found that if ACN content increased from 20% to 50%, the peak height and S/N ratio of R1 increased by 40% and 100%, respectively. However, if ACN content further increased to 80%, the peak height and S/N ratio of R1 decreased dramatically by 200% and 400%, respectively. This result indicated that a higher organic modifier content may not always bring better CAD responses to analytes. Thus, the optimal post-column gradient program was established, where a constant content of 50% ACN was eluted to CAD detector, and the baseline drifting caused by pre-column gradient elution was effectively avoided.
3.3. Validation of HPLC-CAD method
Linearity, LOQ, accuracy, and precision were validated by this developed HPLC-CAD method. The linearities and LOQs were also compared with those results simultaneously acquired at UV 203 nm. The correlation coefficients (r) of most of the saponins were above 0.999, and those of the rest of the analytes were above 0.994, which basically met the requirements of quantification determination. In comparison to CAD results, UV detector generally provided higher correlation coefficients (>0.999). However, LOQs of CAD for most of the saponins were obviously lower than those of UV detector, manifesting a higher sensitivity of CAD compared with UV detector. The RSDs for injection precision were all below 2.6% (n = 3) (Table 1). The developed HPLC-CAD method proved to be accurate by the explanation of recoveries. Mean recoveries of 3 spiked concentration levels for each analyte were ranged from 85.7–112.9%, with the RSDs within 6.8%, which could also be interpreted as intra-day precision. The method validation results of recovery and intra-day precision for each saponin could be found in Table 1S.† These results demonstrated that the HPLC-CAD method was linear, sensitive, accurate and precise for quantification of saponins in notoginseng sample. Although the linearity results of CAD detector were inferior to those of UV detector, this proposed HPLC-CAD method was more sensitive, and was a reliable method for the analysis of saponins in raw and processed notoginseng.| Saponin | RT (min) | Linearity range (mg mL−1) | Calibration curve (CAD, n = 7) | LOQ (CAD, ng) | Calibration curve (UV, n = 7) | LOQ (UV, ng) | Injection precision (RSD, %, n = 3) |
|---|---|---|---|---|---|---|---|
| a More data could be found in the ESI. | |||||||
| R1a | 20.6 | 0.0048–0.3603 | Y = 2.7382X + 0.0047, r = 0.9994 | 40.0 | Y = 0.3783X + 0.0001, r = 0.9995 | 80.1 | 0.52 |
| Rg1 | 29.7 | 0.0142–1.0669 | Y = 2.5478X + 0.0803, r = 0.996 | 118.5 | Y = 0.5167X − 0.0031, r = 0.9999 | 118.5 | 0.28 |
| Re | 31.6 | 0.0024–0.1790 | Y = 3.1448X − 0.0031, r = 0.9998 | 79.5 | Y = 0.3871X − 0.0007, r = 0.9996 | 79.5 | 0.68 |
| Rf | 43.9 | 0.00146–0.1096 | Y = 3.2941X + 0.0015, r = 0.9994 | 24.4 | Y = 0.5165X + 0.0002, r = 0.9994 | 24.4 | 0.20 |
| Rb1 | 47.0 | 0.0104–0.7793 | Y = 1.8034X + 0.0567, r = 0.994 | 86.6 | Y = 0.3362X − 0.0011, r = 1.0000 | 86.6 | 0.17 |
| 20(S)-Rg2 | 48.7 | 0.00224–0.1681 | Y = 3.1435X + 0.0071, r = 0.998 | 18.7 | Y = 0.5569X − 0.0001, r = 0.9999 | 37.4 | 0.96 |
| 20(S)-Rh1 | 49.0 | 0.00291–0.2181 | Y = 3.1826X + 0.0082, r = 0.999 | 24.2 | Y = 0.6615X − 0.0005, r = 0.9999 | 48.5 | 0.12 |
| 20(R)-Rg2 | 49.9 | 0.00247–0.1850 | Y = 3.1004X + 0.0016, r = 0.9992 | 20.6 | Y = 0.5309X − 0.0002, r = 0.9998 | 41.1 | 0.26 |
| 20(R)-Rh1 | 51.0 | 0.00256–0.1919 | Y = 3.1374X + 0.0028, r = 0.9992 | 21.3 | Y = 0.6424X − 0.0005, r = 0.9999 | 42.6 | 0.41 |
| Rb2 | 51.7 | 0.00141–0.1056 | Y = 3.1191X − 0.0050, r = 0.9998 | 23.5 | Y = 0.3619X − 0.0001, r = 0.9998 | 23.5 | 0.90 |
| Rb3 | 52.6 | 0.00284–0.3267 | Y = 3.0678X − 0.0043, r = 0.9997 | 25.0 | Y = 0.3517X − 0.0002, r = 0.9999 | 25.0 | 0.43 |
| F1 | 54.9 | 0.00125–0.0934 | Y = 3.0166X − 0.0034, r = 0.9997 | 20.8 | Y = 0.5650X − 0.0002, r = 0.9999 | 83.0 | 0.12 |
| Rd | 56.5 | 0.00238–0.1787 | Y = 2.6787X + 0.0044, r = 0.998 | 19.9 | Y = 0.4191X − 0.0003, r = 1.0000 | 39.7 | 0.22 |
| F2 | 63.6 | 0.00158–0.1188 | Y = 2.9717X + 0.0014, r = 0.999 | 13.2 | Y = 0.5272X − 0.0003, r = 1.0000 | 26.4 | 0.45 |
| 20(S)-Rg3 | 66.5 | 0.00082–0.0616 | Y = 3.5249X − 0.0012, r = 0.9995 | 13.7 | Y = 0.5836X − 0.0001, r = 1.0000 | 13.7 | 0.37 |
| 20(R)-Rg3 | 67.1 | 0.00057–0.0426 | Y = 3.4383X − 0.0026, r = 0.995 | 9.5 | Y = 0.5329X + 0.0001, r = 0.9991 | 9.5 | 0.56 |
| 20(S)-PPT | 69.2 | 0.00164–0.1230 | Y = 3.2837X + 0.0032, r = 0.999 | 13.7 | Y = 0.9282X − 0.0005, r = 0.9999 | 54.7 | 0.30 |
| CK | 72.8 | 0.00101–0.0757 | Y = 2.9136X − 0.0030, r = 0.9997 | 33.6 | Y = 0.5789X − 0.0000, r = 0.999 | 33.6 | 0.75 |
| 20(S)-Rh2 | 74.3 | 0.00078–0.0581 | Y = 3.9694X − 0.0028, r = 0.9998 | 12.9 | Y = 0.7483X − 0.0003, r = 1.0000 | 25.8 | 1.05 |
| 20(R)-Rh2 | 74.6 | 0.00049–0.0364 | Y = 4.0073X − 0.0036, r = 0.9992 | 8.1 | Y = 0.6942X + 0.0002, r = 1.0000 | 16.2 | 0.24 |
| 20(S)-PPD | 86.0 | 0.00111–0.0831 | Y = 3.6304X − 0.0017, r = 0.9996 | 18.5 | Y = 0.8634X − 0.0005, r = 0.9999 | 36.9 | 0.26 |
| 20(R)-PPD | 86.6 | 0.00066–0.0492 | Y = 3.6493X − 0.0032, r = 0.9996 | 10.9 | Y = 0.8227X − 0.0002, r = 0.9999 | 21.9 | 2.57 |
3.4. Structural identification of saponins in notoginsengs
LC-QTOFMS analysis both in ESI negative and positive mode was utilized for structural identification of the saponins in notoginseng. For those saponins with authentic reference standards available in this experiment, their retention times of chromatographic peaks were further confirmed. The addition of 0.01% formic acid in mobile phase A which facilitated the ionization of saponins would not affect the retention times of TIC chromatographic peaks, and the retention time differences were less than 0.7% for the all saponins with authentic reference standards. Results suggested that ESI negative mode provided stronger mass spectrometric signals for PPD and PPT type saponins under this chromatographic condition. Most of the saponins existed in notoginseng could acquire [M − H]− and/or [M + HCOO]− under ESI negative mode, which provided explicit information on molecular weights of those undetermined saponins. Moreover, mass peaks of m/z 459 and m/z 475, which represent the ion fragments of [PPD − H]− and [PPT − H]−, respectively, always appeared in MS spectrograms in ESI negative mode for PPD and PPT type saponins.The glycosyl substituents in notoginseng mainly include glucose (GLC), xylose (Xyl), arabinose (Ara), rhamnose (Rha), and mannose (Man), etc. For instance, two most characteristic ion peaks for Glc in ESI negative mode are m/z 161, i.e., [(Glc-H2O) − H]−, and m/z 101, which represents the fragment as a result of a neutral loss of C2H4O2 group from m/z 161. Furthermore, the structure transformation of saponins based on collision-induced dissociation provides detailed information on the identification of glycosyl substituents. For example, if there is a loss of m/z162, it usually indicates the loss of a Glc-H2O fragment. Nevertheless, the loss of m/z146, 132 and 324 practically suggest the loss of Rha-H2O, Xyl-H2O or Ara-H2O, and Glc–Glc-2H2O fragment, respectively.
Moreover, ESI positive mode was also a good means for the identification of [M + H]+ and [M + Na]+ for those saponins with fairly weak molecular ion peaks in ESI negative mode. Besides, some mass spectrometric fragments collected in ESI positive mode provided essential clues for the structural confirmation of saponin agylcons. For instance, the existing of a series of fragments including m/z 443, 425 and 407 practically indicate the ion fragments of [PPD-H2O + H]+, [PPD-2H2O + H]+ and [PPD-3H2O + H]+, respectively. However, the occurrence of the series of m/z 441, 423 and 405 imply the ion fragments of [PPT-2H2O + H]+, [PPT-3H2O + H]+ and [PPT-3H2O + H]+, respectively.
Raw and processed notoginseng sample solutions were analyzed by LC-ESI-QTOFMS. At an analytical level, a total of 16 saponins were identified in raw notoginseng, and 25 more saponins were found in processed samples. The structures of the saponins in raw and processed P. notoginseng are given in Fig. 2, and the observed precursor and product ions of saponins are listed in Tables 2 and 3. What should be mentioned is that different medicinal part, including main root, branch root, rhizome and root hair, of notoginseng did not show difference in the saponin components. The differences of saponin molecular weights obtained by MassHunter software and the results inferred from molecular formulas were all below 4 ppm. The structure skeletons of the saponins in notoginseng included PPD, PPT, C-20 dehydrated PPD, C-20 dehydrated PPT, 25-OH PPD, and 25-OH PPT. If the saponins are classified by the aglycons, 16 PPD type saponins were found, where 5 original saponins in raw samples and 11 more secondary saponins in processed samples were identified. Nevertheless, 25 PPT type saponins were discovered, in which 11 original saponins and 24 secondary saponins were figured out.
| No. | Peak identification | Rt (min) | Theoretical accurate mass (m/z) | Experimental (m/z) (ESI−) or (ESI+) | Mass accuracy (ppm) | CID (m/z) |
|---|---|---|---|---|---|---|
| a These compounds have been further confirmed by the peak retention time of authentic reference standard. | ||||||
| 1 | 20-O-Glucoginsenoside Rf | 9.0 | 961.5378[M − H]− | 961.5372[M − H]− | 0.62 | 637.4300[M − H − 2(Glc-H2O)]−, 475.3781[M − H − 3(Glc-H2O)]−, 323.1008[(Glc–Glc)-2H2O − H]−, 161.0459[(Glc-H2O) − H]−, 101.0243[(Glc-H2O) − H − C2H4O2]− |
| 441.3726[PPT-2H2O + H]+, 423.3621[PPT-3H2O + H]+, 405.3516[PPT-4H2O + H]+ | ||||||
| 2 | Notoginsenoside R3 | 15.3 | 961.5378[M − H]− | 961.5372[M − H]− | 0.62 | 799.4883[M − H − (Glc-H2O)]−, 637.4343[M − H − 2(Glc-H2O)]−, 475.3793[M − H − 3(Glc-H2O)]−, 161.0453[(Glc-H2O) − H]−, 101.0241[(Glc-H2O) − H − C2H4O2]− |
| 441.3726[PPT-2H2O + H]+, 423.3622[PPT-3H2O + H]+, 405.3516[PPT-4H2O + H]+ | ||||||
| 3 | Notoginsenoside R1a | 18.0 | 931.5272[M − H]− | 931.5270[M − H]− | 0.21 | 799.4885[M − H − (Xyl-H2O)]−, 637.4349[M − H − (Xyl-H2O) − (Glc-H2O)]−, 475.3835[M − H − (Xyl-H2O) − 2(Glc-H2O)]−, 161.0466[(Glc-H2O) − H]−, 101.0253[(Glc-H2O) − H − C2H4O2]− |
| 4 | Ginsenoside Rg1a | 25.6 | 845.4904[M + HCOO]− | 845.4913[M + HCOO]− | 1.06 | 799.4874[M − H]−, 637.4352[M − H − (Glc-H2O)]−, 475.3816[M − H − 2(Glc-H2O)]−, 161.0455[(Glc-H2O) − H]−, 101.0248[(Glc-H2O) − H − C2H4O2]− |
| 5 | Ginsenoside Rea | 27.3 | 945.5428[M − H]− | 945.5426[M − H]− | 0.21 | 799.4885[M − H − (Rha-H2O)]−, 783.4897[M − H − (Glc-H2O)]−, 637.4327[M − H − (Rha-H2O) − (Glc-H2O)]−, 475.3811[M − H − (Rha-H2O) − 2(Glc-H2O)]−, 101.0263[(Glc-H2O) − H − C2H4O2]− |
| 6 | Malonyl-ginsenoside Rg1 | 34.9 | 885.4853[M − H]− | 885.4855[M − H]− | 0.23 | 799.4822[M − H − Mal]−, 781.4746[M − H − Mal-H2O]−, 679.4442[M − H − (Glc-H2O)]−, 637.4328[M − H − Mal-(Glc-H2O)]−, 475.3798[M − H − Mal-2(Glc-H2O)]−, 161.0451[(Glc-H2O) − H]−, 101.0243[(Glc-H2O) − H − C2H4O2]− |
| 441.3729[PPT-2H2O + H]+, 423.3624[PPT-3H2O + H]+, 405.3517[PPT-4H2O + H]+ | ||||||
| 7 | Yesanchinoside D | 37.1 | 887.5010[M + HCOO]− | 887.5012[M + HCOO]− | 0.23 | 841.4956[M − H]−, 799.4872[M − H − COCH2]−, 781.4737[M − H − COCH2-H2O]−, 637.4217[M − H − COCH2-(Glc-H2O)]−, 619.4226[M − H − COCH2-(Glc-H2O)-H2O]−, 475.3801[M − H − COCH2-2(Glc-H2O)]−, 161.0453[(Glc-H2O) − H]−, 101.0246[(Glc-H2O) − H − C2H4O2]− |
| 441.3727[PPT-2H2O + H]+, 423.3622[PPT-3H2O + H]+, 405.3517[PPT-4H2O + H]+ | ||||||
| 8 | Notoginsenoside R4 | 41.2 | 1239.6379[M − H]− | 1239.6373[M − H]− | 0.48 | 1107.5932[M − H − (Xyl-H2O)]−, 1077.5821[M − H − (Glc-H2O)]−, 945.5419[M − H − (Xyl-H2O) − (Glc-H2O)]−, 783.4915[M − H − (Xyl-H2O) − 2(Glc-H2O)]−, 621.4379[M − H − (Xyl-H2O) − 3(Glc-H2O)]− |
| 443.3885[PPD-H2O + H]+, 425.3777[PPD-2H2O + H]+, 407.3674[PPD-3H2O + H]+, 325.1128[(Glc–Glc)-2H2O + H]− | ||||||
| 9 | Notoginsenoside Fa | 43.1 | 1239.6379[M − H]− | 1239.6372[M − H]− | 0.56 | 1107.5947[M − H − (Xyl-H2O)]−, 945.5433[M − H − (Xyl-H2O) − (Glc-H2O)]−, 783.4858[M − H − (Xyl-H2O) − 2(Glc-H2O)]−, 621.4373[M − H − (Xyl-H2O) − 3(Glc-H2O)]−, 161.0452[(Glc-H2O) − H]−, 101.0241[(Glc-H2O) − H − C2H4O2]− |
| 443.3886[PPD-H2O + H]+, 425.3779[PPD-2H2O + H]+, 407.3674[PPD-3H2O + H]+, 325.1129[(Glc–Glc) + H − 2H2O]+ | ||||||
| 10 | 20(S)-Notoginsenoside R2 | 45.0 | 769.4744[M − H]− | 769.4748[M − H]− | 0.52 | 637.4339[M − H − (Xyl-H2O)]− 475.3789[M − H − (Xyl-H2O) − (Glc-H2O)]−, 161.0449[(Glc-H2O) − H]−, 101.0244[(Glc-H2O) − H − C2H4O2]− |
| 459.3834[PPT-H2O + H]+, 441.3728[PPT-2H2O + H]+, 423.3625[PPT-3H2O + H]+, 405.3517[PPT-4H2O + H]+ | ||||||
| 11 | Ginsenoside Rb1a | 45.8 | 1107.5957[M − H]− | 1107.5960[M − H]− | 0.27 | 945.5443[M − H − (Glc-H2O)]−, 927.5267[M − H − (Glc-H2O)-H2O]−, 783.4933[M − H − 2(Glc-H2O)]−, 765.4803[M − H − 2(Glc-H2O)-H2O]−, 621.4370[M − H − 3(Glc-H2O)]−, 459.3871[M − H − 4(Glc-H2O)]−, 423.4257[M − H − 4(Glc-H2O)-2H2O]−, 323.1072[(Glc-Glc)-2H2O − H]− |
| 12 | 20(S)-Ginsenoside Rg2a | 47.5 | 829.4955[M − HCOO]− | 829.4962[M − HCOO]− | 0.84 | 783.4931[M − H]−, 637.4324[M − H − (Rha-H2O)]−, 475.3810[M − H − (Rha-H2O) − (Glc-H2O)]−, 161.0451[(Glc-H2O) − H]−, 101.0245[(Glc-H2O) − H − C2H4O2]− |
| 13 | 20(S)-Ginsenoside Rh1a | 47.9 | 683.4376[M + HCOO]− | 683.4387[M + HCOO]− | 1.61 | 637.4359[M − H]−, 475.3820[M − H − (Glc-H2O)]−, 161.0450[(Glc-H2O) − H]−, 101.0250[(Glc-H2O) − H − C2H4O2]− |
| 14 | Ginsenoside F1a | 53.6 | 683.4376[M + HCOO]− | 683.4385[M + HCOO]− | 1.31 | 637.4334[M − H]−, 475.3790[M − H − (Glc-H2O)]−, 161.0442[Glc-H2O − H]−, 101.0250[(Glc-H2O) − H − C2H4O2]− |
| 661.4266[M + Na]+ | 661.4290[M + Na]+ | 3.63 | 459.3834[PPT-H2O + H]+, 441.3728[PPT-2H2O + H]+, 423.3623[PPT-3H2O + H]+, 405.3517[PPT-4H2O + H]+ | |||
| 15 | Ginsenoside Rda | 55.4 | 945.5428[M − H]− | 945.5430[M − H]− | 0.21 | 783.4950[M − H − (Glc-H2O)]−, 621.4379[M − H − 2(Glc-H2O)]−, 161.0464[(Glc-H2O) − H]−, 101.0247[(Glc-H2O) − H − C2H4O2]− |
| 443.3855[PPD-H2O + H]+, 425.3784[PPD-2H2O + H]+, 407.3677[PPD-3H2O + H]+, 325.1129[(Glc–Glc) + H − 2H2O]+ | ||||||
| 16 | Gypenoside-XVIIa | 57.4 | 945.5428[M − H]− | 945.5426[M − H]− | 0.21 | 621.4857[M − H − 2(Glc-H2O)]−, 475.8270[M − H − 3(Glc-H2O)]−, 323.0988[(Glc–Glc)-2H2O]−, 161.0456[(Glc-H2O) − H]−, 101.0242[(Glc-H2O) − H − C2H4O2]− |
| 443.3887[PPD-H2O + H]+, 425.3779[PPD-2H2O + H]+, 407.3674[PPD-3H2O + H]+, 325.1128[(Glc–Glc) + H − 2H2O]+ | ||||||
| No. | Peak identification | Rt (min) | Theoretical accurate mass (m/z) | Experimental (m/z) (ESI−) or (ESI+) | Mass accuracy (ppm) | CID (m/z) |
|---|---|---|---|---|---|---|
| a The peaks at retention times of 68.1 min and 68.5 min are a pair of PPT type saponin isomers, with a –Rha glycosyl substitute in their structures. | ||||||
| 17 | 20(S)-25-OH ginsenoside Rh1 | 11.8 | 701.4482[M − HCOO]− | 701.4490[M − HCOO]− | 1.14 | 655.4433[M − H]−, 493.3905[M − H − (Glc-H2O)]−, 161.0454[(Glc-H2O) − H]−, 101.0245[(Glc-H2O) − H − C2H4O2]− |
| 639.4479[M + H − H2O]+, 477.3942[M + H − H2O-(Glc-H2O)] +, 459.3841[PPT-H2O + H]+, 441.3730[PPT-2H2O + H]+, 423.3623[PPT-3H2O + H]+, 405.3518[PPT-4H2O + H]+ | ||||||
| 18 | 20(R)-25-OH ginsenoside Rh1 | 15.0 | 701.4482[M − HCOO]− | 701.4492[M − HCOO]− | 1.43 | 655.4427[M − H]−, 493.3907[M − H − (Glc-H2O)]−, 161.0453[(Glc-H2O) − H]−, 101.0246[(Glc-H2O) − H − C2H4O2]− |
| 639.4481[M + H − H2O]+, 477.3942[M + H − H2O-(Glc-H2O)]]+, 441.3730[PPT-2H2O + H]+, 423.3623[PPT-3H2O + H]+, 405.3521[PPT-4H2O + H]+ | ||||||
| 19 | 20(S)-Rh1 (Man as glycosyl substituent) | 39.4 | 683.4376[M − HCOO]− | 683.4385[M − HCOO]− | 1.32 | 637.4332[M − H]−, 475.3811[M − H − (Man-H2O)]−, 161.0455[Man − H]−, 101.0242[Man − H-C2H4O2]− |
| 20 | 20(R)-Rh1 (Man as glycosyl substituent) | 40.5 | 683.4376[M − HCOO]− | 683.4384[M − HCOO]− | 1.17 | 637.4333[M − H]−, 475.3771[M − H − (Man-H2O)]−, 161.0447[Man − H]−, 101.0235[Man − H − C2H4O2]− |
| 21 | 20(R)-Ginsenoside Rg2* | 48.4 | 829.4955[M − HCOO]− | 829.4953[M − HCOO]− | 0.24 | 783.4926[M − H]−, 637.4548[M − H − (Rha-H2O)]−, 161.0444[Man − H]− |
| 807.4870[M + Na]+, 639.4472[M + H − (Rha-H2O)]+, 477.3940[M + H − (Rha-H2O) − (Glc-H2O)]+, 441.3729[PPT-2H2O + H]+, 423.3626[PPT-3H2O + H]+, 405.3519[PPT-4H2O + H]+ | ||||||
| 22 | 20(R)-Ginsenoside Rh1* | 49.5 | 683.4376[M − HCOO]− | 683.4386[M − HCOO]− | 1.346 | 637.4361[M − H]−, 475.3836[M − H − (Glc-H2O)]−, 161.0456[(Glc-H2O) − H]−, 101.0248[(Glc-H2O) − H − C2H4O2]− |
| 661.4294[M + Na]+, 621.4369[M + H − H2O]+, 603.4265[M + H − 2H2O]+, 459.3834[M + H − H2O-(Glc-H2O)]+, 441.3731[PPT-2H2O + H]+, 423.3629[PPT-3H2O + H]+, 405.3519[PPT-4H2O + H]+ | ||||||
| 23 | 25-OH ginsenoside Rg3 | 50.9 | 801.5006[M − H]− | 801.5008[M − H]− | 0.25 | 639.4473[M − H − (Glc-H2O)]−, 477.3960[M − H − 2(Glc-H2O)]−, 101.0245[(Glc-H2O) − H − C2H4O2]− |
| 785.5064[M + H − H2O]+, 767.4949[M + H − 2H2O]+, 749.4846[M + H − 3H2O]+, 623.4524[M + H − H2O-(Glc-H2O)]+, 461.3992[M + H − H2O-2(Glc-H2O)]+, 443.3884[PPD + H − H2O]+, 425.3780[PPD + H − 2H2O]+, 407.3674[PPD + H − 3H2O]+, 325.1987[Glc–Glc-2H2O + H]+ | ||||||
| 24 | Gypenoside LXXV | 60.5 | 783.4900[M − H]− | 783.4904[M − H]− | 0.51 | 621.4378[M − H − (Glc-H2O)]−, 459.3802[M − H − 2(Glc-H2O)]−, 161.0451[(Glc-H2O) − H]−, 101.0246[(Glc-H2O) − H − C2H4O2]− |
| 785.5046[M + H]+ | 785.5063[M + H]+ | 2.16 | 623.4525[M + H − (Glc-H2O)]+, 461.3991[M + H − 2(Glc-H2O)]+, 443.3877[PPD + H − H2O]+, 425.3775[PPD + H − 2H2O]+, 407.3674[PPD + H − 3H2O]+, 325.1136[(Glc–Glc)-2H2O + H]+ | |||
| 25 | Gypenoside LXXV isomer | 61.2 | 783.4900[M − H]− | 783.4902[M − H]− | 0.26 | 621.4403[M − H − (Glc-H2O)]−, 161.0441[(Glc-H2O) − H]−, 101.0252[(Glc-H2O) − H − C2H4O2]− |
| 785.5046[M + H]+ | 785.5057[M + H]+ | 1.40 | 623.4523[M + H − (Glc-H2O)]+, 461.3986[M + H − 2(Glc-H2O)]+, 443.3875[PPD + H − H2O]+, 425.3775[PPD + H − 2H2O]+, 407.3673[PPD + H − 3H2O]+, 325.1132[(Glc–Glc)-2H2O + H]+ | |||
| 26 | Notoginsenoside T5 | 61.5 | 751.4638[M − H]− | 751.4641[M − H]− | 0.40 | 619.4225[M − H − (Xyl-H2O)]−, 457.3687[M − H − (Xyl-H2O) − (Glc-H2O)]−, 161.0452[(Glc-H2O) − H]−, 101.0247[(Glc-H2O) − H − C2H4O2]− |
| 27 | Notoginsenoside U | 62.2 | 799.4849[M − H]− | 799.4820[M − H]− | 3.63 | |
| 801.4995[M + H]+ | 801.4994[M + H]+ | 0.12 | 477.3940[M + H − 2(Glc-H2O)]+, 459.3833[PPT-H2O + H]+, 441.3730[PPT-2H2O + H]+ | |||
| 28 | Notoginsenoside T5 isomer | 62.4 | 751.4638[M − H]− | 751.4642[M − H]− | 0.53 | 619.4218[M − H − (Xyl-H2O)]−, 161.0463[(Glc-H2O) − H]− |
| 441.3728[PPT-2H2O + H]+, 423.3624[PPT-3H2O + H]+, 405.3514[PPT-4H2O + H]+ | ||||||
| 29 | Ginsenoside F4 | 62.8 | 765.4795[M − H]− | 765.4797[M − H]− | 0.26 | 619.4268[M − H − (Rha-H2O)]−, 161.0460[(Glc-H2O) − H]−, 101.0246[(Glc-H2O) − H − C2H4O2]− |
| 789.4759[M + Na]+ | 789.4765[M + Na]+ | 0.86 | 621.4333[M + H − (Rha-H2O)]−, 441.3728[PPT-2H2O + H]+, 423.3624[PPT-3H2O + H]+, 405.3514[PPT-4H2O + H]+ | |||
| 30 | Ginsenoside RK3 | 63.4 | 665.4270[M + HCOO]− | 665.4280[M + HCOO]− | 1.50 | 619.4590[M − H]−, 457.3739[M − H − (Glc-H2O)]−, 457.3739[PPT-H2O]−, 161.0452[(Glc-H2O) − H]−, 101.0246[(Glc-H2O) − H − C2H4O2]− |
| 621.4361[M + H]+ | 621.4368[M + H]+ | 1.13 | 603.4264[M + H − H2O]+,441.3731[M + H − H2O-(Glc-H2O)]+, 441.3731[PPT-2H2O + H]+, 423.3629[PPT-3H2O + H]+, 405.3521[PPT-4H2O + H]+ | |||
| 31 | Ginsenoside Rh4 | 64.4 | 665.4270[M + HCOO]− | 665.4280[M + HCOO]− | 1.50 | 619.4200[M − H]−, 457.3702[M − H − (Glc-H2O)]−, 457.3702[PPT-H2O]−, 161.0452[Glc-H2O − H]−, 101.0248[Glc-H2O − H − C2H4O2]− |
| 621.4361[M + H]+ | 621.4365[M + H]+ | 0.64 | 603.4263[M + H − H2O]+, 441.3732[M + H − H2O-(Glc-H2O)]+, 441.3732[PPT-2H2O + H]+, 423.3629[PPT-3H2O + H]+, 405.3520[PPT-4H2O + H]+ | |||
| 32 | 20(S)-Ginsenoside Rg3* | 65.4 | 783.4900[M − H]− | 783.4905[M − H]− | 0.64 | 621.4385[M − H − (Glc-H2O)]−, 459.3867[M − H − 2(Glc-H2O)]−, 323.1867[(Glc–Glc)-2H2O − H]−, 161.0458[(Glc-H2O) − H]−, 101.0248[(Glc-H2O) − H − C2H4O2]− |
| 33 | 20(R)-Ginsenoside Rg3* | 65.9 | 783.4900[M − H]− | 783.4904[M − H]− | 0.51 | 621.4392[M − H − (Glc-H2O)]−, 459.3870[M − H − 2(Glc-H2O)]−, 161.0449[(Glc-H2O) − H]−, 101.0248[(Glc-H2O) − H − C2H4O2]− |
| 34 | Unknown 1a | 68.1 | 975.7623[M + Na]+ | 975.7642[M + Na]+ | 1.95 | 953.7817[M + H]+, 499.3755[M + H − PPT]+, 477.394[PPT + H − H2O]+, 459.3840[PPT + H − H2O]+, 441.3736[PPT + H − 2H2O]+, 423.3629[PPT + H − 3H2O]+, 405.3523[PPT + H − 4H2O]+, 147.1167[(Rha-H2O) + H]+ |
| 805.9862[M − H − (Rha-H2O)]−, 475.3794[PPT − H]− | ||||||
| 35 | Unknown 2a | 68.5 | 975.7623[M + Na]+ | 975.7639[M + Na]+ | 1.64 | 953.7817[M + H]+, 499.3764[M + H − PPT]+, 459.3840[PPT + H − H2O]+, 441.3736[PPT + H − 2H2O]+, 423.3628[PPT + H − 3H2O]+, 405.3520[PPT + H − 4H2O]+, 147.1167[(Rha-H2O) + H]+ |
| 805.9878[M − H − (Rha-H2O)]−, 475.3808[PPT − H]− | ||||||
| 36 | Ginsenoside RK1 | 71.4 | 765.4795[M − H]− | 765.4801[M − H]− | 0.78 | 603.4288[M − H − (Glc-H2O)]−, 161.04534[(Glc-H2O) − H]−, 101.0246[(Glc-H2O) − H − C2H4O2]− |
| 789.4770[M + Na]+, 767.4950[M + H]+, 605.4417[M + H − (Glc-H2O)]+, 587.4312[M + H − (Glc-H2O)-H2O]+, 477.3947[PPT + H]+, 459.3835[PPT + H − H2O]+, 443.3887[M + H − 2(Glc-H2O)]+, 443.3887[PPD + H − H2O]+, 425.3784[PPD + H − 2H2O]+, 407.3677[PPD + H − 3H2O]+, 325.1131[2(Glc-H2O) + H]+ | ||||||
| 37 | Ginsenoside Rg5 | 71.9 | 765.4795[M − H]− | 765.4803[M − H]− | 1.05 | 603.4277[M − H − (Glc-H2O)]−, 323.0994[(Glc–Glc)-2H2O + H]+, 161.0454[(Glc-H2O) − H]−, 101.0245[(Glc-H2O) − H − C2H4O2]− |
| 789.4770[M + Na]+, 767.4950[M + H]+, 605.4416[M + H − (Glc-H2O)]+, 587.4315[M + H − (Glc-H2O)-H2O]+, 477.3947[PPT + H]+, 459.3836[PPT + H − H2O]+, 443.3886[M + H − 2(Glc-H2O)]+, 443.3886[PPD + H − H2O]+, 425.3784[PPD + H − 2H2O]+, 407.3678[PPD + H − 3H2O]+, 325.1131[(Glc–Glc)-2H2O) + H]+ | ||||||
| 38 | 20(S)-Ginsenoside Rh2* | 73.2 | 667.4427[M − HCOO]− | 667.4438[M − HCOO]− | 1.65 | 621.4388[M − H]−, 459.3904[M − H − (Glc-H2O)]−, 161.0458[(Glc-H2O) − H]−, 101.0241[(Glc-H2O) − H − C2H4O2]− |
| 39 | 20(R)-Ginsenoside Rh2* | 73.6 | 667.4427[M − HCOO]− | 667.4439[M − HCOO]− | 1.80 | 621.4385[M − H]−, 459.3869[M − H − (Glc-H2O)]−, 161.0459[(Glc-H2O) − H]−, 101.0242[(Glc-H2O) − H − C2H4O2]− |
| 40 | Ginsenoside RK2 | 80.0 | 649.4321[M − HCOO]− | 649.4331[M − HCOO]− | 1.54 | 603.4266[M − H]−, 161.0457[(Glc-H2O) − H]− |
| 605.4412[M + H]+ | 605.4392[M + H]+ | 3.30 | 443.3892[M + H − (Glc-H2O)]+, 443.3892[PPD + H − H2O]+, 425.3787[PPD + H − 2H2O]+, 407.3682[PPD + H − 3H2O]+ | |||
| 41 | Ginsenoside Rh3 | 80.6 | 649.4321[M − HCOO]− | 649.4329[M − HCOO]− | 1.23 | 603.4292[M − H]−, 161.0457[(Glc-H2O) − H]−, 101.0239[(Glc-H2O) − H − C2H4O2]− |
| 605.4412[M + H]+ | 605.4412[M + H]+ | 0.00 | 443.3888[M + H − (Glc-H2O)]+, 443.3888[PPD + H − H2O]+, 425.3785[PPD + H − 2H2O]+, 407.3681[PPD + H − 3H2O]+ | |||
During the processing of notoginseng, the two most common routes to produce secondary saponins were (1) deglycosylation and (2) dyhydration at C-20 of their aglycons. For example, the deglycosylation of one –Glc at C-20 of ginsenoside Rd forms Rg3, and the loss of one –Glc at C-3 of Rg3 produces Rh2. Moreover, notoginsenoside T5, ginsenoside F4, Rk3/Rh4, RK1/Rg5, and Rk2/Rh3 are the C-20 dehydrated products of 20(S)-notoginsenoside R2, 20(S)-ginsenoside Rg2, 20(S)/(R)-Rh1, 20(S)/(R)-Rg3 and 20(S)/(R)-Rh2, respectively.
3.5. Impact of structural features on CAD responses of PPD and PPT ginsenosides
Since the intercept value in linearity test was far smaller than the corresponding slope value for both CAD (<3%) and UV (<0.6%) detector, the slope could be regarded as the RF for each analyte. In virtue to its feature of being a universal detector where the RF value is theoretically independent of the analyte's chemical structure, CAD presented far smaller RF differences than UV detector. However, there was still little variation of the CAD RFs of each saponin in the process of nebulization in CAD detector, leading to a narrow range of CAD RFs. Based on the difference of CAD RFs of authentic reference standards, the rules of CAD response over structural features of PPD and PPT ginsenosides were discovered. Nevertheless, UV RFs were not found to have relevance to saponin structures.It was found that the optical rotations at C-20 had no influence on CAD responses (Table 2Sa†). 20(S)-Epimers of Rg2, Rh1, Rg3, Rh2 and PPD all exhibited very little variation (<2.3%) on CAD RFs compared with their corresponding 20(R)-epimers. Data also showed that CAD RFs of PPD saponins were generally higher (6–26%) than those of PPT saponins (Table 2Sb†). The glycosyl substituent at C-3 position of PPD saponins had little impact on CAD RFs (Table 2Sc†). For example, 20(S)-PPD, 20(S)-Rh2 and 20(S)-Rg3 all bear –H at C-20, and the substituents at C-3 are –H, –Glc, and –Glc–Glc, respectively. The variations of CAD RFs of these above three saponins were below 15%. If these variations are ignored, the CAD RFs of all PPD saponins with the same C-20 substituent while different C-3 substituents could be considered as a constant value.
The glycosyl substituent at C-6 position of PPT saponins had a little influence on CAD RFs (Table 2Sd†). For example, when –H is fixed at C-20, the variations of CAD RFs of 20(S)-PPT, Rh1, Rf, and Rg2, which bears –H, –Glc, –Glc–Glc and –Glc–Rha at C-6, respectively, were within 5%. However, if –Glc is fixed at C-20, cases were complicated. When C-6 substituent changed from –H to –Glc, CAD RF decreased by 18%. When C-6 substituent changed from –Glc to –Glc–Xyl, i.e., one more five-carbon sugar was added, CAD RF increased by 7%. When C-6 substituent changed from –Glc to –Glc–Rha, i.e., one more six-carbon sugar was added, CAD RF increased by 23%.
For PPT saponins, once the C-6 substituent was fixed, the change of C-20 substituent from –H to –Glc caused less than 25% of the variation of CAD RFs (Table 2Se†). Nevertheless, different glycosyl substituents at C-20 caused relatively greater changes speaking of PPD saponins. It indicated that once C-3 substituent is fixed, the adding of one more six-carbon sugar, i.e. –Glc, causd the reduction of CAD from 25% to 49%. Furthermore, the addition of one more five-carbon sugar, i.e., –Xyl and –Ara, led to a 72% increase of CAD RFs (Table 2Sf†).
3.6. Prediction of CAD RF values of saponins with known structure
Now that the impact of structural features of PPD and PPT saponins on their CAD RFs has been discovered, the CAD RF values of those saponins without authentic reference standards available could be predicted. The assigned RF values were further employed to determine the overall saponin content in raw and processed notoginseng samples. To make things easier, PPT saponins can be divided into two groups according to their CAD RF values: (1) high polar saponins, including R1 and Rg1, with retention times prior to 30 min; (2) medium and low polar saponins, including F1, Rg2, Rh1, Re, Rf and PPT, with retention times from 30 min to 69 min. CAD RFs in the former group were 2.55 and 2.74, with the variation less than 8%. Nevertheless, CAD RFs in the latter group were ranging from 3.02 to 3.29, with the variation within 10%. This classification made the CAD RF assignments easier when PPT saponins were concerned: if the saponin was of high polarity (Rt < 30 min), CAD RF was assigned to be 2.64, i.e., the average value of 2.55 and 2.74; if the polarity of the saponin was medium or low (Rt > 30 min), CAD RF was assigned as 3.16, i.e., the average value of 3.02 and 3.29. Since C-20 dehydrated and 25-OH hydrated PPD and PPT saponins all belong to secondary saponins which may only appear in processed notoginsengs with quite low contents, their authentic reference standards were difficult to obtain. The assignment rule of CAD RFs of these PPD/PPT saponin derivatives was regarded as the same as regular PPD/PPT saponins. The prediction of CAD RF values of the saponins without authentic reference standard in raw and processed notoginsengs is presented in Tables 4 and 5.| RT (min) | Saponin | Aglycon | C-3 substituent | C-6 substituent | C-20 substituent | Predicted CAD RF | Comments on the prediction of CAD RF |
|---|---|---|---|---|---|---|---|
| a Notoginsenoside R4 has one more –Xyl at C-6 substituent compared with Rb1. Since the addition of one –Xyl to C-6 in PPD ginsenosides causes 15% increasing of CAD RF, the CAD RF of R4 was assigned as 2.07 (=1.80 × 115%).b Notoginsenoside Fa has one more –Xyl at C-3 substituent compared with Rb1. Since the glycosyl substituent at C-3 position of PPD type ginsenosides had little impact on CAD RF, the CAD RF of Fa was assigned as that of Rb1.c Gypenoside-XVII has one more –Glc at C-20 substituent compared with F2. Since the change of –Glc to –Glc–Glc at C-20 in PPD ginsenosides causes 50% decreases of CAD RF, the CAD RF of gypenoside-XVII was assigned as 1.98 (=2.97÷150%). | |||||||
| 10 | 20-O-Glucoginsenoside Rf | PPT | –H | –Glc2–1Glc | –Glc | 2.64 | High polar PPT type ginsenoside |
| 17 | Notoginsenoside R3 | PPT | –H | –Glc | –Glc6–1Glc | 2.64 | |
| 36.9 | Malonyl-ginsenoside Rg1 | PPT | –H | –Glc6–1Malony | –Glc | 3.16 | Medium/low polar PPT type ginsenoside |
| 38.4 | Yesanchinoside D | PPT | –H | –Glc6–Ac | –Glc | 3.16 | |
| 42.3 | Notoginsenoside R4 | PPD | –Glc2–1Glc | — | –Glc6–1Glc6–1Xyl | 2.07 | a |
| 44.2 | Notoginsenoside Fa | PPD | –Glc2–1Glc2–1Xyl | — | –Glc6–1Glc | 1.80 | b |
| 46.1 | Notoginsenoside 20(S)-R2 | PPT | –H | –Glc2–1Glc | –H | 3.16 | Medium/low polar PPT type ginsenoside |
| 58 | Gypenoside XVII | PPD | –Glc | — | –Glc6–1Glc | 1.98 | c |
| RT (min) | Saponin | Aglycon | C-3 substituent | C-6 substituent | C-20 substituent | Predicted CAD RF | Comments on the prediction of CAD RF |
|---|---|---|---|---|---|---|---|
| a Gypenoside LXXV has one more –Glc at C-20 substituent compared with CK. Since the change of –Glc to –Glc–Glc to C-20 in PPD ginsenosides causes 50% decreasing of CAD RF, the CAD RF of gypenoside LXXV was assigned as 1.94 (=2.91÷150%).b Ginsenoside RK1 and Rg5 are C-20 dehydrated Rg3.c Ginsenoside RK2 and Rh3 are C-20 dehydrated Rh2. | |||||||
| 13.3 | 25-OH-20(S)-Rh1 | 25-OH PPT | –H | –Glc | –H | 2.64 | High polar PPT ginsenoside |
| 17.0 | 25-OH-20(R)-Rh1 | 25-OH PPT | –H | –Glc | –H | 2.64 | |
| 40.3 | 20(S)-Rh1 isomer | PPT | –H | –Mannose | –H | 3.16 | Medium/low polar PPT ginsenoside |
| 41.4 | 20(R)-Rh1 isomer | PPT | –H | –Mannose | –H | 3.16 | |
| 52.3 | 25-OH Rg3 | 25-OH PPD | –Glc | — | –Glc | 3.48 | The average RF value of 20(S)-Rg3 and 20(R)-Rg3 |
| 59.3 | Gypenoside LXXV | PPD | –H | — | –Glc6–1Glc | 1.94 | a |
| 61.5 | Gypenoside LXXV isomer | PPD | –H | — | –Glc–Glc (linkage not sure) | 1.94 | |
| 62.3 | Notoginsenoside T5 | C-20 dehydrated PPT | –H | –Glc2–1Xyl | –H | 3.16 | Medium/low polar PPT ginsenoside |
| 63.2 | Notoginsenoside U | PPT | –H | –H | –Glc6–1Glc | 3.16 | |
| 63.4 | Notoginsenoside T5 isomer | C-20 dehydrated PPT | –H | –Glc2–1Xyl | –H | 3.16 | |
| 63.9 | Ginsenoside F4 | C-20 dehydrated PPT | –H | –Glc2–1Rha | –H | 3.16 | |
| 64.5 | Ginsenoside RK3 | C-20 dehydrated PPT | –H | –Glc | –H | 3.16 | |
| 65.4 | Ginsenoside Rh4 | C-20 dehydrated PPT | –H | –Glc | –H | 3.16 | |
| 69.1 | Unknown 1 | PPT | –H | Unknown | Unknown | 3.16 | |
| 69.5 | Unknown 2 | PPT | –H | Unknown | Unknown | 3.16 | |
| 72.4 | Ginsenoside RK1 | C-20 dehydrated PPD | –Glc2–1Glc | — | –H | 3.48 | The average RF value of 20(S)-Rg3 and 20(R)-Rg3b |
| 73.1 | Ginsenoside Rg5 | C-20 dehydrated PPD | –Glc2–1Glc | — | –H | 3.48 | |
| 81.0 | Ginsenoside RK2 | C-20 dehydrated PPD | –Glc | — | –H | 3.99 | The average RF value of 20(S)-Rh2 and 20(R)-Rh2 c |
| 81.5 | Ginsenoside Rh3 | C-20 dehydrated PPD | –Glc | — | –H | 3.99 | |
3.7. Validation results of the prediction of CAD RFs
The predicted CAD RFs values for notoginsenoside R1 and ginsenoside Rg1 are 2.64, since they are both high polar PPT type saponins. And the predicted CAD RFs for the medium or low polar PPT type saponins with authentic reference standard, i.e., ginsenoside Re, Rf, 20(S)-/20(R)-Rh1, 20(S)-/20(R)-Rg2, F1, and 20(S)-PPT, are all assigned as 3.16. The differences between the predicted RFs and CAD slopes of linear regression for all the ten PPT saponins are between 0.48% and 4.75%, indicating the accuracy of our prediction. Moreover, the retention time of gypenoside XVII was 58.9 min, which was in accordance with the retention time, i.e., 58.0 min, based on our identification by the combination of LC-QTOFMS and HPLC-CAD analysis (Table 2). The linear regression equation was Y = 2.0744X + 0.00818, r = 0.999 (n = 6). The difference between the CAD slope of linear regression and the predicted RF value was 4.77%, suggesting that our prediction was reasonable and accurate. Although it is very difficult to get all the authentic reference standards in raw and processed notoginsengs at this stage, our prediction is a quite easy, accurate and stable method to determine the complex saponin contents in this herb according to our validation results.3.8. Determination of saponins in raw and processed notoginseng
Notoginseng saponins are the main component of P. notoginseng. Besides, volatile oils, polysaccharides, dencichine and flavonoids are also contained in this herb. Among these species, volatile oils do not have CAD signals because CAD can only detect non-volatile or semi-volatile compounds. Polysaccharides and dencichine are of high polarity, and may have very short retention times or could even hardly be retained on C18 column. Moreover, the contents of flavonoids are quite low in the underground parts of notoginseng. As long as the assigned saponin peaks without authentic reference standards in CAD chromatograms are one-to-one corresponding to the ones identified by LC-QTOFMS, the low content of flavonoids would not interfere with the detection of saponins in this method.The content of saponins in raw notoginseng from different cultivated places, and of various sizes and medicinal parts were calculated. Moreover, saponin content in processed notoginseng with diverse processing procedures were also evaluated and compared. The powder (80 mesh) of corresponding notoginseng sample was employed to prepare sample solutions. For the saponins with authentic reference standards available in this experiment, a simple internal standard method was performed to calculate the content of saponins. However, if the saponin had no authentic reference standard, its CAD RF was predicted and calculated based on the structure identified using LC-QTOFMS. And then an internal standard method could easily be carried out. To better compare the saponin content in different kinds of notoginsengs, the water content of each batch of notoginseng powder was previously determined by Karl Fisher titration, and the final results were calculated based on water-free basis. The saponins contents in raw notoginsengs are listed in Table 3S.† Our results conformed to the data presented in previous literature.30
The total amount of all saponins, the total amount of ginsenoside Rg1, Rb1 and R1, and the ratio of PPD vs. PPT saponins were compared in raw P. notoginseng. Take 120 head raw P. notoginseng as an example, the saponins with content greater than 1 mg g−1 were as follows: R1, Rg1, Re, malonyl Rg1, R4, Fa, 20(S)-R2, Rb1, 20(S)-Rh1 and Rd, among which R1, Rg1 and Rb1 are considered to be the three most representative saponins, since the total amount of R1, Rg1 and Rb1 is used to evaluate the quality of raw P. notoginseng in Chinese Pharmacopoeia (Chp). The total amount of R1, Rg1 and Rb1 accounted for 74% to 81% of the total saponin content no matter of what herbal size, cultivate place or medicinal part, with fairly low variation (RSD 3.0%, n = 15). It can be concluded that the total amount of these three saponins can be used to represent the total saponin content, and tedious determination of total amount is unnecessary. In Chp, the total amount of these three saponins should be no less than 5.0% (50 mg g−1). Based on our results, all but the saponin contents in the main root of countless head notoginseng met the requirement in Chp. Interestingly, our study exhibited that the saponin contents were not always proportionate to the size of main root. To our surprise, 40-head, not 20 or 30-head, P. notoginseng possessed the highest total saponin content no matter where the cultivated place was. In addition, 40-head P. notoginseng also exhibited the highest PPD/PPT ratios, which were 0.984 and 0.912 for the notoginseng from Yunnan and Guangxi, respectively. The PPD/PPT ratios in main root of P. notoginseng from Yunnan were basically higher than those from Guangxi for the corresponding sizes greater than 120 heads. Literature has mentioned that PPD/PPT ratio could be used as a tool to distinguish the types of ginseng.31 Thus, we tried to find the relationship between PPD/PPT ratios and total saponin contents. We correlated these two results obtained from different heads of notoginsengs (Yunnan), a correlation coefficient of 0.614 was calculated from the linear regression (Fig. 1S†). Although the linear correlation was not good enough, there is still some trend that the PPD/PPT ratio has relation to total saponins at least in the case of different size of main root. This result suggested that PPD/PPT ratio could be regarded as a parameter to determine the quality of P. notoginseng. Moreover, the total saponin amount in different medicinal parts decreased in the following order: 40 or 60 head main root ≈ rhizome > branch root ≫ root hair. What should be mentioned is that the total amount of R1, Rg1 and Rb1 in root hair was 52.5 mg g−1, which was only 5% above the qualified line of notoginseng in Chp 2010. These results conformed to the description of Sanqi in Chp 2010, in which only the main root, branch root and rhizome are included.
Chan et al. firstly introduced the term “biomarker” into steamed notoginseng.32 Here, the “biomarker” means the compounds only existed in steamed notoginseng, or those of quite high content in steamed notoginseng yet of extremely low content in raw herbs. The concept of biomarker could be successfully utilized to differentiate raw and processed notoginseng. In our study, 25 secondary saponins were found in processed notoginseng, among which 20(R)-Rh1, Rk3, Rh4, 20(S)-/20(R)-Rg3, RK1 and Rg5 were those with the highest amount. In the processed notoginseng which has been steamed for 3 h, the content of these above saponins were no less than 0.5 mg kg−1. Thus, these 7 saponins were designated as biomarkers in processed notoginseng at the analytical level in this experiment. Researches have shown that ginsenoside Rk3, Rh4, Rg3, RK1 and Rg5 are proven to be biologically potent in anti-tumor activities and in cardiovascular systems.33–41 Thus, the function difference of processed notoginseng compared with the raw herbs was basically due to the difference of compound basis, in which the biomarkers may have major contributions to the pharmacological activities of processed notoginseng.
Steaming is a most frequently used processing method for this herb, and steaming at 100 °C for 3 h has been set as the provincial standard for processed notoginseng powder in Yunnan, China, since Apr. 1, 2013. The contents of the biomarkers and total secondary saponins increased basically with the increasing of steaming time, and the data of the content of all saponins are shown in Table 4Sa–e.† However, the increasing rate of the biomarker content in steamed notoginseng from 3 h to 4 h was not obvious, indicating that a 3 to 4 h steaming time is enough, while a longer steaming time may not always lead to significantly greater contents of secondary saponins. Thus, steaming for 3 h can be regarded as the beginning of the platform of a relatively constant content of biomarkers. Furthermore, the ratio of secondary vs. original saponins of 3 h-steamed notoginsengs was the highest among the steamed samples, proving that 3 h could be regarded as the best steaming time for notoginseng based on our results. Moreover, steaming is also a cost efficient way for processing notoginseng.
Except for steaming, frying and stewing of notoginseng are two other traditional processing procedures in Chinese culture. However, our results showed that the contents of biomarkers and total secondary saponins were quite low compared with those in 3 h-steamed notoginseng, indicating that frying or stewing may not be an appropriate way for processing notoginseng. In recent literatures, baking and autoclaving are two techniques to process notoginsengs.42,43 Apparently, with the increasing of temperature and time, the contents of biomarkers and secondary saponins increased dramatically. Given the same temperature (100 °C) and processing time (24 h), the contents of biomarkers and secondary saponins in autoclaved notoginseng were of about 10 times compared with those in the baked sample, indicating that pressure was an important parameter for the generation of secondary saponins. According to our data, the biomarkers and secondary saponin contents in baked notoginseng under 100 °C for as long as 48 h were just comparable to those in 3 h-steamed sample, suggesting that the humidity in the processing procedure was also essential. Thus, four major parameters, i.e., humidity, temperature, time and pressure, should be taken into consideration on the journey of seeking for the best processing procedure for notoginseng. The saponin contents in the processed notoginsengs which have been baked at 120 °C for 24 h, autoclaved at 100 °C for 4 to 6 h, and autoclaved at 120 °C for 2 h are comparable to those in 3 h-steamed notoginsengs. Although baking is easy to achieve, a relatively longer processing time leads to a low cost efficiency in this case. In the case of autoclaving, the 6 h-autoclaving at 120 °C and the 18 h-autoclaving at 100 °C led to an increasing of the amount of secondary saponins by 3 to 5 times. However, the advantage of steaming at high pressure at 100 °C over ordinary steaming for a relatively short period of processing time, i.e., less than 6 h, was not very obvious. The autoclaving at 120 °C for a comparatively shorter period could produce a considerable amount of secondary saponins. However, the equipment of autoclave needs special attention for operation, and could not be implemented in household. To sum up, steaming for 3 h was confirmed to be an easy and cost efficient method for the processing of notoginseng. Nevertheless, autoclaving for a relatively longer period of time could be an economic and efficient way to prepare and isolate secondary saponins with potent pharmacological effects which are not existed in raw notoginsengs.
4. Conclusions
In this study, an in-depth analysis of the saponin components and saponin contents in raw and processed Panax notoginseng was performed. A gradient eluted HPLC method using acetonitrile and water as mobile phases coupled with charged aerosol detector was established and validated to determine 22 PPD and PPT saponins and aglycons simultaneously in notoginseng. Since the discrepancy of CAD RFs of saponins is quite narrow, the impact of the structural features, including the type of aglycon, the optical rotations at C-20, the glycosyl substituent and the glycosyl linkage of different PPD and PPT saponins on their CAD RFs was discovered. Moreover, the structures of saponins existed in raw and processed notoginseng were extensively identified using LC-QTOFMS. At the analytical level, 16 original and 25 secondary saponins were detected in raw and processed notoginseng, respectively. Since the saponins in raw or processed notoginsengs were predominately dammarane-type tetracyclic triterpenoid saponins, the impact rules were successfully applied to predict the CAD RFs of saponins, and then a simple internal standard method could be carried out to determine the content of each saponin in notoginsengs. An investigation on saponin contents in raw P. notoginseng of different sizes, growing places, medicinal parts, and those in processed notoginseng of different processing procedures, i.e., steaming, baking, autoclaving, stewing and frying was then implemented. Our results indicated that 40 head main root of notoginseng possessed the greatest quantity of total saponins among raw herbs. The PPD vs. PPT ratio in raw P. notoginseng main roots from Yunnan were greater that those from Guangxi for the corresponding size greater than 120 heads. Moreover, the total saponins in different medicinal parts were decreased in the following order: 40 or 60 head main root ≈ rhizome > branch root ≫ root hair. Ginsengnoside 20(R)-Rh1, Rk3, Rh4, 20(S)-/20(R)-Rg3, RK1 and Rg5 were set as biomarkers, as these were the 6 most abundant secondary saponins existed in processed notoginseng, and may be responsible for the main difference of pharmacological functions between the raw and processed herbs. As a result, steaming for 3 h was proven to be the best processing method as it produces fairly high amount of biomarkers and secondary saponins in processed samples for a relatively shorter period and in a cost effective and convenient way. The authentic reference standards of quite a few saponins in notoginseng could not be obtained commercially, especially those secondary saponins in processed herb. The major advantage of this HPLC-CAD method over previous established QAMS methods is that it is not necessary to get relative correction factors for each saponin experimentally, but the CAD response factor could just be predicted theoretically according to our data. Thus, this article has for the first time provided an easy and reliable method to evaluate the content of those saponins and the quality of raw and processed notoginseng which had never been extensively studied in previous literatures.Abbreviations
| Ara | Arabinose |
| CAD | Charge aerosol detector |
| Chp | Chinese Pharmacopoeia |
| ESI | Electrospray ion |
| Glc | Glucose |
| Man | Mannose |
| P. notoginseng | Panax notoginseng |
| PPD | Protopanaxadiol |
| PPT | Protopanaxatriol |
| QAMS | Multi-component with single marker |
| QTOFMS | Quadrupole time-of-flight mass spectrometry |
| RCF | Relative correction factor |
| RF | Response factor |
| Rha | Rhamnose |
| Xyl | Xylose |
Acknowledgements
This work was financially supported by the Project of Shanghai Committee of Science and Technology (13401900300) and the Foundation of the Ministry of Education of China (NCET-10-0944).References
- S. Y. Han, H. X. Li, X. Ma, K. Zhang, Z. Z. Ma, Y. Jiang and P. F. Tu, Evaluation of the anti-myocardial ischemia effect of individual and combined extracts of Panax notoginseng and Carthamustinctorius in rats, J. Ethnopharmacol., 2013, 145, 722 CrossRef CAS PubMed
.
- P. W. Wang, J. G. Cui, X. Y. Du, Q. B. Yang, C. L. Jia, M. Q. Xiong, X. T. Yu, L. Li, W. J. Wang, Y. Chen and T. Zhang, Panax notoginseng saponins (PNS) inhibits breast cancer metastasis, J. Ethnopharmacol., 2014, 154, 663 CrossRef CAS PubMed
.
- W. Xia, C. H. Sun, Y. Zhao and L. J. Wu, Hypolipidemic and antioxidant activities of Sanchi (Radix Notoginseng) in rats fed with a high fat diet, Phytomedicine, 2011, 18, 516 CrossRef CAS PubMed
.
- C. Y. Yang, J. Wang, Y. Zhao, L. Shen, X. Jiang, Z. G. Xie, N. Liang, L. Zhang and Z. H. Chen, Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components, J. Ethnopharmacol., 2010, 130, 231 CrossRef CAS PubMed
.
- State Administration of Traditional Chinese Medicine (People’s Republic of China), Sanqi, Zhong Hua Ben Cao Jing Hua Ben, Shanghai Science and Technology Publishers, Shanghai, China, 1998, vol. 1, pp. 1305–1319 Search PubMed
.
- D. F. Toh, D. N. Patel, E. C. Y. Chan, A. Teo, S. Y. Neo and H. L. Koh, Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells, Chin. Med., 2011, 6, 4 CrossRef PubMed
.
- S. Sun, C. Z. Wang, R. Tong, X. L. Li, A. Fishbein, Q. Wang, T. C. He, W. Du and C. S. Yuan, Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities, Food Chem., 2010, 118, 307 CrossRef CAS
.
- S. Sun, L. W. Qi, G. J. Du, S. R. Mehendale, C. Z. Wang and C. S. Yuan, Red notoginseng: Higher ginsenoside content and stronger anticancer potential than Asian and American ginseng, Food Chem., 2011, 125, 1299 CrossRef CAS PubMed
.
- A. J. Lau, D. F. Toh, T. K. Chua, Y. K. Pang, S. O. Woo and H. L. Koh, Antiplatelet and anticoagulant effects of Panax notoginseng: Comparison of raw and steamed Panax notoginseng with Panax ginseng and Panax quinquefolium, J. Ethnopharmacol., 2009, 125, 380 CrossRef CAS PubMed
.
- C. Z. Wang, E. McEntee, S. Wicks, J. A. Wu and C. S. Yuan, Phytochemical and analytical studies of Panax notoginseng (Burk.), J. Nat. Med., 2006, 60, 97 CrossRef CAS
.
- C. Q. Wang, X. H. Jia, S. Zhu, K. Komatsu, X. Wang and S. Q. Cai, A systematic study on the influencing parameters and improvement of quantitative analysis of multi-component with single marker method using notoginseng as research subject, Talanta, 2015, 134, 587 CrossRef CAS PubMed
.
- C. J. S. Lai, T. Tan, S. L. Zeng, L. R. Xu, L. W. Qi, E. H. Liu and P. Li, An enzymatic protocol for absolute quantification of analogues: application to specific protopanoxadiol-type ginsenosides, Green Chem., 2015, 17, 2580 RSC
.
- C. J. S. Lai, T. Tan, S. L. Zeng, X. Dong, E. H. Liu and P. Li, Relative quantification of multi-components in Panax notoginseng (Sanqi) by high-performance liquid chromatography with mass spectrometry using mobile phase compensation, J. Pharm. Biomed. Sci., 2015, 102, 150 CrossRef CAS PubMed
.
- R. W. Dixon and D. S. Peterson, Development and testing of a detection method for liquid chromatography based on aerosol charging, Anal. Chem., 2002, 74, 2930 CrossRef CAS PubMed
.
- U. Holzgrabe, C. J. Nap and S. Almeling, Control of impurities in L-aspartic acid and L-alanine by high-performance liquid chromatography coupled with a corona charged aerosol detector, J. Chromatogr. A, 2010, 1217, 294 CrossRef CAS PubMed
.
- Z. Long, Z. M. Guo, X. D. Liu, Q. Zhang, X. g. Liu, Y. Jin, L. N. Liang, H. S. Li, J. Wei and N. P. Wu, A sensitive non-derivatization method for apramycin and impurities analysis using hydrophilic interaction liquid chromatography and charged aerosol detection, Talanta, 2016, 146, 423 CrossRef CAS PubMed
.
- I. Marquez-Sillero, S. Cardenas and M. Valcarcel, Determination of water-soluble vitamins in infant milk and dietary supplement using a liquid chromatography on-line coupled to a corona-charged aerosol detector, J. Chromatogr. A, 2013, 1313, 253 CrossRef CAS PubMed
.
- M. Plaza, J. Kaiuki and C. Turner, Quantification of individual phenolic compounds' contribution to antioxidant capacity in apple: a novel analytical tool basedon liquid chromatography with diode array, electrochemical, and charged aerosol detection, J. Agric. Food Chem., 2014, 62, 409 CrossRef CAS PubMed
.
- M. Poplawska, A. Blazewicz, K. Bukowinska and Z. Fijalek, Application of high-performance liquid chromatography with charged aerosol detection for universal quantitation of undeclared phosphodiesterase-5 inhibitors in herbal dietary supplements, J. Pharm. Biomed. Anal., 2013, 84, 232 CrossRef CAS PubMed
.
- L. M. Nair and J. O. Werling, Aerosol based detectors for the investigation of phos-pholipid hydrolysis in a pharmaceutical suspension formulation, J. Pharm. Biomed. Anal., 2009, 49, 95 CrossRef CAS PubMed
.
- M. Poplawska, A. Blazewicz, P. Zolek and Z. Fijalek, Determination of flibanserinand tadalafil in supplements for women sexual desire enhancement usinghigh-performance liquid chromatography with tandem mass spectrometer, diode array detector and charged aerosol detector, J. Pharm. Biomed. Anal., 2014, 94, 45 CrossRef CAS PubMed
.
- Q. Zhou, M. T. Chen, L. H. Zhu and H. Q. Tang, Determination of perfluorinated carboxylic acids in water using liquid chromatography coupled to a corona-charged aerosol detector, Talanta, 2015, 136, 35 CrossRef CAS PubMed
.
- L. Wang, W. S. He, H. X. Yan, Y. Jiang, K. S. Bi and P. F. Tu, Performance evaluation of charged aerosol and evaporative light scattering detection for the determination of ginsenosides by LC, Chromatographia, 2009, 70, 603 CAS
.
- C. C. Bai, S. Y. Han, X. Y. Chai, Y. Jiang, P. Li and P. F. Tu, Sensitive determination of saponins in Radix et rhizoma notoginseng by charged aerosol detector coupled with HPLC, J. Liq. Chromatogr. Relat. Technol., 2008, 32, 242 CrossRef
.
- A. Stojanovic, M. Lammerhofer, D. Kogelnig, S. Schiesel, M. Sturm, M. Galanski, R. Krachler, B. Keppler and W. Lindner, Analysis of quaternary ammoniumand phosphonium ionic liquids by reversed-phase high-performance liquidchromatography with charged aerosol detection and unified calibration, J. Chromatogr. A, 2008, 1209, 179 CrossRef CAS PubMed
.
- P. H. Gamache, R. S. McCarthy, S. M. Freeto, D. J. Asa, M. J. Woodcock, K. Laws and R. O. Cole, HPLC analysis of non-volatile analytes using charged aerosol detection, LC·GC Eur., 2005, 18, 345 CAS
.
- S. Matsuyama, Y. Orihara, S. Kinugasa and H. Ohtani, Effects of densities of brominated flame retardants on the detection response for HPLC analysis with acorona-charged aerosol detector, Anal. Sci., 2015, 31, 61 CrossRef CAS PubMed
.
- L. E. Magnusson, D. S. Risley and J. A. Koropchak, Aerosol-based detectors for liquid chromatography, J. Chromatogr. A, 2015, 1421, 68 CrossRef CAS PubMed
.
- S. Almeling, D. Ilko and U. Holzgrabe, Charged Aerosol detection in pharmaceutical analysis, J. Pharm. Biomed. Anal., 2012, 69, 50 CrossRef CAS PubMed
.
- S. P. Li, C. F. Qiao, Y. W. Chen, J. Zhao, X. M. Cui, Q. W. Zhang, X. M. Liu and D. J. Hu, A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food, J. Chromatogr. A, 2013, 1331, 302 CrossRef PubMed
.
- C. Y. Liu, J. G. Song, P. F. Li, H. S. Yu and F. X. Jin, Ginsenoside contents in three different ginseng, Journal of Dalian Polytechnic University, 2011, 30, 79–82 CAS
.
- E. C. Y. Chan, S. L. Yap and A. J. Lau, Ultra-performance liquid chromatography/time-of-flight mass spectrometry based metabolomics of raw and steamed Panax notoginseng, Rapid Commun. Mass Spectrom., 2007, 21, 519 CrossRef CAS PubMed
.
- H. H. Kwok, G. L. Guo, J. K. Lau, Y. K. Cheng, J. R. Wang, Z. H. Jiang, M. H. Keung, N. K. Mak, P. Y. K. Yue and R. N. S. Wong, Stereoisomers ginsenosides-20(S)-Rg3 and -20(R)-Rg3 differentially induce angiogenesis through peroxisome proliferator-activated receptor-gamma, Biochem. Pharmacol., 2012, 83, 893 CrossRef CAS PubMed
.
- R. Wu, Q. Ru, L. Chen, B. Ma and C. Li, Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice, J. Food Sci., 2014, 79, H1430 CrossRef CAS PubMed
.
- J. G. Lee, Y. Y. Lee, S. Y. Kim, J. S. Pyo, H. S. Yun-Choi and J. H. Park, Platelet antiaggregating activity of ginsenosides isolated from processed ginseng, Pharmazie, 2009, 64, 602 CAS
.
- H. K. Ju, J. G. Lee, M. K. Park, S. J. Park, C. H. Lee, J. H. Park, C. H. Lee, J. H. Park and S. W. Kwon, Metabolomic investigation of the anti-platelet aggregation activity of ginsenoside Rk1 reveals attenuated 12-HETE production, J. Proteome Res., 2012, 11, 4939 CrossRef CAS PubMed
.
- J. S. Kim, E. J. Joo, J. Chun, Y. W. Ha, J. H. Lee, Y. Han and Y. S. Kim, Induction of apoptosis by ginsenoside Rk1 in SK-MEL-2-human melanoma, Arch. Pharmacal Res., 2012, 35, 717 CrossRef CAS PubMed
.
- Y. S. Maeng, S. Maharjan, J. H. Kim, J. H. Park, Y. Y. Suk and Y. M. Kim, et al., Rk1, a ginsenoside, is a new blocker of vascular leakage acting through actin structure remodeling, PLoS One, 2013, 8, e68659 CAS
.
- J. Sun, G. Sun, X. Meng, H. Wang, M. Wang, M. Qin, B. Ma, Y. Luo, Y. Yu, R. Chen, Q. Ai and X. Sun, Ginsenoside RK3 prevents hypoxia-reoxygenation induced apoptosis in H9c2 cardiomyocytes via AKT and MAPK pathway, J. Evidence-Based Complementary Altern. Med., 2013, 690190 Search PubMed
.
- N. I. Baek, D. S. Kim, Y. H. Lee, J. D. Park, C. B. Lee and S. I. Kim, Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng, Planta Med., 1996, 62, 86 CrossRef CAS PubMed
.
- S. J. Kim and A. K. Kim, Anti-breast cancer activity of Fine Black ginseng (Panax ginseng Meyer) and ginsenoside Rg5, J. Ginseng Res., 2015, 39, 125e134 Search PubMed
.
- D. F. Toh, L. S. New, H. L. Koh and E. C. Y. Chan, Ultra-high performance liquid chromatography/time-of-flight mass spectrometry (UHPLC/TOFMS) for time-dependent profiling of raw and steamed Panax notoginseng, J. Pharm. Biomed. Anal., 2010, 52, 43 CrossRef CAS PubMed
.
- D. Wang, P. Y. Liao, H. T. Zhu, K. K. Chen, M. Xu, Y. J. Zhang and C. R. Yang, The processing of Panax notoginseng and the transformation of its saponin components, Food Chem., 2012, 132, 1808 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03193e |
| This journal is © The Royal Society of Chemistry 2016 |