DOI:
10.1039/C6RA03165J
(Paper)
RSC Adv., 2016,
6, 35609-35616
Convenient synthesis of functionalized pyrrolo[3,4-b]pyridines and pyrrolo[3,4-b]quinolines via three-component reactions†
Received
3rd February 2016
, Accepted 2nd April 2016
First published on 5th April 2016
Abstract
A simple protocol for convenient construction of pyrrolo[3,4-b]pyridine skeleton was successfully developed by base promoted three-component reaction of β-enamino imide, aromatic aldehydes and malononitrile as well as its ester and amide derivatives. The similar three-component reaction of β-enamino imide, aromatic aldehydes and cyclic diketones such as dimedone and cyclohexane-1,3-dione afforded functionalized pyrrolo[3,4-b]quinolines in good yields.
Introduction
Pyrrolo[3,4-b]pyridine is the core structure of many pharmacological agents and natural alkaloids displaying promising biological activities as indole and purine bioisosteres.1 Several pyrrolo[2,3-b]pyridines have been reported as selective inhibitors of different isoforms of Janus kinase (JAK).2 Particularly, moxifloxacin is one of the most widely used fourth generation fluoroquinolones antibacterial agents with well tolerated drug developed by Bayer AG.3 As a consequence, many efficient synthetic methods and elegant procedures have been developed for the construction of pyrrolo[3,4-b]pyridine system and its functionalized derivatives.4–7 The reported synthetic methods for pyrrolo[2,3-b]pyridines were mostly started from the substituted pyridine precursors, while the syntheses starting from pyrrole precursors were not attracted much attention. Recently, we reported that β-enamino ester generated in situ from the addition of arylamine to dialkyl acetylenedicarboxylate reacted with aromatic aldehydes and malononitrile to give polysubstituted 1,4-dihydropyridine derivatives.8 In these years, the reactive β-enamino esters generated from the addition of aliphatic and aromatic amines to electron-deficient alkynes have been emerged as one of most valuable tools for the preparation of structurally diverse carbocyclic and heterocyclic molecules.9–12 In this respect, β-enamino imide, which could be easily formed from the sequential reaction of dimethyl acetylenedicarboxylate with arylamine and methylamine,13 maintains the structural character of β-enaminone and β-enamino ester, is also proved to be one of potential synthons for construction of diverse heterocyclic systems.14 In continuation of our aim of developing domino multicomponent reaction for versatile nitrogen-containing heterocyclic systems,15,16 herein we wish to provide one efficient synthetic procedure for the synthesis of functionalized pyrrolo[3,4-b]pyridines and pyrrolo[3,4-b]quinolines by the three-component reactions of 3-arylamino-1-methyl-1H-pyrrole-2,5-diones with aromatic aldehydes and reactive methylene compounds such as malononitrile and dimedone.
Results and discussion
According to our previously established reaction conditions for the synthesis of 1,4-dihydropyridines by the reaction of aromatic aldehydes, malononitrile and β-enamino esters,8a,13d an equivalent amount of 3-arylamino-1-methyl-1H-pyrrole-2,5-dione, aromatic aldehyde and malononitrile in ethanol in the presences of triethylamine as base catalyst was refluxed for about five hours, the expected pyrrolo[3,4-b]pyridines 1a–1j were obtained in satisfactory yields (Table 1, entries 1–10). It should be pointed out that the reaction afforded very pure products after filtration and no need further separation with chromatography. When ethyl cyanoacetate and cyanoacetamide were employed in the reaction, the corresponding functionalized pyrrolo[3,4-b]pyridines 1k–1p were also obtained in good yields (Table 1, entries 11–17). These results indicated that this three-component reaction has a wide variety of substrates and with high efficiency. The structures of the products 1a–1p were fully characterized by spectroscopic methods. The single crystal structures of the compounds 1a (Fig. 1) and 1p (Fig. S1†) were determined by X-ray diffraction method. It is interesting to find that 1,4-dihydropyridyl ring exists in a slightly distortional plane and the two aryl groups at 1,4-positions stretched to the same side of ring.
Table 1 Synthesis of 4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridines 1a–1qa

|
| Entry |
Compd |
R (Ar) |
Ar′ |
E |
Yieldb (%) |
| Reaction conditions: β-enamino imide (0.5 mmol), aromatic aldehyde (0.5 mmol) and malononitrile (0.5 mmol), Et3N (0.5 mmol), EtOH (15.0 mL), reflux, 5 h. Isolated yields. |
| 1 |
1a |
Bn |
p-CH3C6H4 |
CN |
90 |
| 2 |
1b |
Bn |
p-CH3OC6H4 |
CN |
88 |
| 3 |
1c |
Bn |
p-ClC6H4 |
CN |
81 |
| 4 |
1d |
p-CH3C6H4 |
p-CH3C6H4 |
CN |
92 |
| 5 |
1e |
p-CH3C6H4 |
p-ClC6H4 |
CN |
65 |
| 6 |
1f |
p-CH3C6H4 |
p-BrC6H4 |
CN |
70 |
| 7 |
1g |
p-ClC6H4 |
C6H5 |
CN |
72 |
| 8 |
1h |
p-ClC6H4 |
p-ClC6H4 |
CN |
95 |
| 9 |
1i |
p-BrC6H4 |
C6H5 |
CN |
85 |
| 10 |
1j |
p-BrC6H4 |
p-ClC6H4 |
CN |
75 |
| 11 |
1k |
p-CH3C6H4 |
m-NO2C6H4 |
CO2Et |
62 |
| 12 |
1l |
p-ClC6H4 |
m-NO2C6H4 |
CO2Et |
52 |
| 13 |
1m |
p-BrC6H4 |
m-NO2C6H4 |
CO2Et |
78 |
| 14 |
1n |
Bn |
C6H5 |
CONH2 |
55 |
| 15 |
1o |
Bn |
p-ClC6H4 |
CONH2 |
68 |
| 16 |
1p |
p-CH3C6H4 |
p-ClC6H4 |
CONH2 |
85 |
| 17 |
1q |
p-BrC6H4 |
p-ClC6H4 |
CONH2 |
82 |
 |
| | Fig. 1 ORTEP-drawings of the crystal structures of 1a. | |
For further demonstrating the synthetic values of β-enamino imide, the similar three-component reaction of 3-arylamino-1-methyl-1H-pyrrole-2,5-dione, aromatic aldehydes and dimedone was also investigated. When the reaction was carried out in ethanol with triethylamine, DABCO, DBU and K2CO3 as base promoter, the reaction resulted in complicate mixture of products. Then, some acid was employed in the reaction. After careful examination, we were pleased to find that the chain products 2a and 2b can be obtained in good yields when the reaction is conducted in ethanol at room temperature by using acetic acid as promoter (Scheme 1). The single crystal structure of the compound 2b was successfully determined by X-ray diffraction method (Fig. 2). The products 2a and 2b obviously came from the addition of β-enamino imide to the in situ generated arylidene dimedone. However, the further annulation process was not accomplished under the above mentioned reaction conditions.
 |
| | Scheme 1 Synthesis of chain products 2a and 2b from three-component reaction. | |
 |
| | Fig. 2 ORTEP-drawings of the crystal structures of 2b. | |
In order to get the annulation product, the harsh reaction conditions were employed in the three-component reaction. When the three-component reaction was carried out with acetic acid both as catalyst and solvent at about 100 °C for ten hours, all cyclization reaction proceeded smoothly and the expected functionalized pyrrolo[3,4-b]quinolines 3a–3j were obtained in moderate to good yields (Table 2). The structures of pyrrolo[3,4-b]quinolines 3a–3j were established by HRMS, IR, 1H and 13C NMR spectra, and were further confirmed by single-crystal X-ray diffraction studies performed for compounds 3a (Fig. 3), 3g and 3j (Fig. S2 and S3†).
Table 2 Synthesis of 5,6,7,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline 3a–3ja
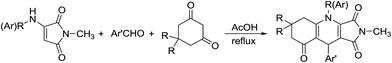
|
| Entry |
Compd |
Ar |
Ar′ |
R |
Yieldb (%) |
| Reaction conditions: β-enamino imide (0.5 mmol), aromatic aldehyde (0.5 mmol), dimedone (0.5 mmol), AcOH (10.0 mL), reflux, 10 h. Isolated yields. |
| 1 |
3a |
p-CH3C6H4 |
C6H5 |
H |
74 |
| 2 |
3b |
p-CH3C6H4 |
p-CH3C6H4 |
CH3 |
80 |
| 3 |
3c |
p-CH3C6H4 |
p-(CH3)3CC6H4 |
CH3 |
66 |
| 4 |
3d |
p-CH3C6H4 |
p-NO2C6H4 |
CH3 |
74 |
| 5 |
3e |
p-CH3C6H4 |
p-NO2C6H4 |
H |
78 |
| 6 |
3f |
p-CH3OC6H4 |
C6H5 |
H |
70 |
| 7 |
3g |
p-CH3OC6H4 |
p-NO2C6H4 |
CH3 |
72 |
| 8 |
3h |
p-ClC6H4 |
p-CH3C6H4 |
H |
72 |
| 9 |
3i |
p-ClC6H4 |
p-NO2C6H4 |
CH3 |
75 |
| 10 |
3j |
p-BrC6H4 |
p-CH3C6H4 |
H |
68 |
 |
| | Fig. 3 ORTEP-drawings of the crystal structures of 3a. | |
Another common cyclic 1,3-dikeone, 4-hydroxy-2H-chromen-2-one, was also employed in the three-component reaction. The chain products 4a and 4b could be obtained in good yields when the three-component reaction was carried out in ethanol at room temperature with acetic acid as catalyst (Scheme 2). However, when the reaction was carried out in refluxing acetic acid, the expected annulated cyclic products did not formed and the chain products were observed to decompose slowly to give complicate mixture. We could not get the desired cyclized products after testing many different reaction conditions. This result displays that the sequential cyclization process is very sensitive to the structure of the substrates and reaction conditions.
 |
| | Scheme 2 Synthesis of chain products 4a and 4b from three-component reaction. | |
For explaining the reaction mechanism, a tandem reaction mechanism is proposed in Scheme 3. In acidic system, aromatic aldehyde condensates with dimedone to give arylidene dimedone (A). Then, nucleophilic addition of β-enamino imide to intermediate (A) results in a open chain product 2. Finally, the intramolecular condensation of reaction of amino group with one of carbonyl group in product 2 affords 1H-pyrrolo[3,4-b]quinoline (3). Obviously, the three-component reaction for formation of pyrrolo[3,4-b]pyridine 1 has similar reaction mechanism as showed in Scheme 3.
 |
| | Scheme 3 Proposed reaction mechanism for three-component reaction. | |
Conclusion
In summary, we have successfully developed convenient procedures for the synthesis of functionalized pyrrolo[3,4-b]pyridines and pyrrolo[3,4-b]quinolines via the three-component reaction of β-enamino imides with aromatic aldehydes and reactive methylene compounds such as malononitrile and dimedone. The advantages of this reaction included using readily available starting substrates, milder reaction conditions, simple separation process, satisfactory yields and diverse molecular outcome. This reaction also explored the versatile applications of reactive synthons of β-enaminone and its ester, amide derivatives for the synthesis of heterocycles. The potential uses of the reaction in synthetic and medicinal chemistry might be quite significant.
Experimental section
1. General procedure for the synthesis of functionalized pyrrolo[3,4-b]pyridines 1a–1q
A mixture of 3-arylamino-1-methyl-1H-pyrrole-2,5-dione (0.5 mmol), aromatic aldehyde (0.5 mmol), malononitrile (0.5 mmol, 0.033 g) and triethylamine (0.5 mmol, 0.050 g) in ethanol (15.0 mL) was refluxed for five hours. TLC monitor indicated that the reaction has finished. After removing the solvent by rotator evaporation at reduced pressure, the resulting solid was titrated by alcohol to give the pure product for analysis.
2-Amino-1-benzyl-4-(4-methoxyphenyl)-6-methyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1a). Yellow solid, 0.176 g, 88%, mp 198–200 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.41–7.37 (m, 2H, ArH), 7.33–7.31 (m, 1H, ArH), 7.26 (d, J = 7.6 Hz, 2H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 6.80 (d, J = 8.0 Hz, 2H, ArH), 6.38 (s, 2H, NH2), 5.54 (d, J = 16.4 Hz, 1H, CH), 5.29 (d, J = 16.4 Hz, 1H, CH), 4.44 (s, 1H, CH), 3.72 (s, 3H CH3), 2.78 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.7, 165.7, 158.7, 152.7, 138.3, 137.3, 136.5, 129.1, 128.9, 128.0, 127.4, 121.7, 114.2, 113.0, 62.2, 55.5, 46.6, 36.0, 23.8; IR (KBr) ν: 3458, 3337, 3241, 2896, 2189, 1764, 1708, 1666, 1564, 1509, 1429, 1380, 1250, 1172, 1110, 1034, 980, 844, 751 cm−1; MS (m/z): HRMS (ESI) calcd for C23H20N4NaO3 ([M + Na]+): 423.1428. Found: 423.1437.
2-Amino-1-benzyl-6-methyl-5,7-dioxo-4-(p-tolyl)-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1b). Yellow solid, 0.173 g, 90%, mp 210–212 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.40–7.31 (m, 3H, ArH), 7.28–7.26 (m, 2H, ArH), 7.05 (d, J = 7.6 Hz, 2H, ArH), 6.95 (d, J = 7.6 Hz, 2H, ArH), 6.39 (s, 2H, NH2), 5.55 (d, J = 16.4 Hz, 1H, CH), 5.29 (d, J = 16.4 Hz, 1H, CH), 4.44 (s, 1H, CH), 2.78 (s, 3H CH3), 2.25 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.6, 165.7, 152.8, 141.4, 138.5, 137.3, 136.6, 129.4, 129.1, 128.0, 127.7, 127.4, 121.6, 112.9, 62.1, 46.6, 36.4, 23.8, 21.0; IR (KBr) ν: 3438, 3358, 3253, 2190, 1765, 1706, 1669, 1554, 1510, 1428, 1381, 1234, 1172, 1111, 1060, 984, 954, 844, 788, 763, 743 cm−1; MS (m/z): HRMS (ESI) calcd for C23H20N4NaO2 ([M + Na]+): 407.1487. Found: 407.1485.
2-Amino-1-benzyl-4-(4-chlorophenyl)-6-methyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1c). Yellow solid, 0.164 g, 81%, mp 207–209 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.41–7.37 (m, 2H, ArH), 7.34–7.31 (m, 3H, ArH), 7.26 (d, J = 7.6 Hz, 2H, ArH), 7.12 (d, J = 7.6 Hz, 2H, ArH), 6.47 (s, 2H, NH2), 5.53 (d, J = 16.4 Hz, 1H, CH), 5.32 (d, J = 16.4 Hz, 1H, CH), 4.55 (s, 1H, CH), 2.78 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.5, 165.5, 153.0, 143.2, 138.9, 137.2, 132.1, 129.7, 129.1, 128.8, 128.0, 127.3, 121.5, 112.1, 61.4, 46.6, 36.2, 23.8; IR (KBr) ν: 3440, 3360, 3252, 3044, 2945, 2852, 2187, 1764, 1706, 1668, 1551, 1428, 1381, 1239, 1171, 1095, 985, 837, 736 cm−1; MS (m/z): HRMS (ESI) calcd for C22H17N4NaO2 ([M + Na]+): 427.0932. Found: 427.0931.
2-Amino-6-methyl-5,7-dioxo-1,4-di-p-tolyl-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1d). Yellow solid, 0.177 g, 92%, mp 243–245 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.33–7.28 (m, 4H, ArH), 7.27–7.25 (m, 2H, ArH), 7.18–7.16 (m, 2H, ArH), 5.54 (s, 2H, NH2), 4.55 (s, 1H, CH), 2.66 (s, 3H CH3), 2.39 (s, 3H, CH3), 2.30 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 163.8, 152.2, 141.7, 139.5, 138.0, 136.7, 132.8, 130.5, 129.6, 128.0, 121.4, 111.3, 61.1, 36.7, 23.6, 21.3, 21.1; IR (KBr) ν: 3470, 3029, 2875, 2178, 1772, 1717, 1671, 1613, 1553, 1508, 1385, 1285, 1185, 1074, 982, 839, 761 cm−1; MS (m/z): HRMS (ESI) calcd for C23H20N4NaO2 ([M + Na]+): 407.1478. Found: 407.1487.
2-Amino-4-(4-chlorophenyl)-6-methyl-5,7-dioxo-1-(p-tolyl)-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1e). Yellow solid, 0.131 g, 65%, mp 246–248 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.43 (s, 4H, ArH), 7.31 (s, 4H, ArH), 5.62 (s, 2H, NH2), 4.65 (s, 1H, CH), 2.67 (s, 3H CH3), 2.39 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.8, 163.7, 152.3, 143.4, 139.6, 138.4, 132.6, 132.2, 130.5, 130.1, 129.6, 128.9, 121.2, 110.4, 60.5, 36.5, 23.6, 21.3; IR (KBr) ν: 3436, 3027, 2874, 2176, 1776, 1717, 1670, 1661, 1551, 1503, 1385, 1327, 1282, 1187, 1087, 983, 840, 791 cm−1; MS (m/z): HRMS (ESI) calcd for C22H17ClN4NaO2 ([M + Na]+): 427.0932. Found: 427.0933.
2-Amino-4-(4-bromophenyl)-6-methyl-5,7-dioxo-1-(p-tolyl)-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1f). Yellow solid, 0.157 g, 70%, mp 243–245 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.58 (d, J = 7.6 Hz, 2H, ArH), 7.38 (d, J = 7.6 Hz, 2H, ArH), 7.31 (s, 4H, ArH), 5.64 (s, 2H, NH2), 4.65 (s, 1H, CH), 2.68 (s, 3H CH3), 2.40 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.8, 163.7, 152.3, 143.9, 139.6, 138.4, 132.6, 131.9, 130.5, 130.4, 129.6, 121.2, 120.8, 110.4, 60.4, 36.6, 23.6, 21.3; IR (KBr) ν: 3470, 3025, 2876, 2177, 1775, 1717, 1672, 1611, 1553, 1505, 1384, 1283, 1187, 1072, 985, 838, 703 cm−1; MS (m/z): HRMS (ESI) calcd for C22H17BrN4NaO2 ([M + Na]+): 471.0427. Found: 471.0424.
2-Amino-1-(4-chlorophenyl)-6-methyl-5,7-dioxo-4-phenyl-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1g). Yellow solid, 0.140 g, 72%, mp 240–242 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.56 (d, J = 8.8 Hz, 2H, ArH), 7.48 (d, J = 8.8 Hz, 2H, ArH), 7.40–7.35 (m, 4H, ArH), 7.29–7.25 (m, 1H, ArH), 5.79 (s, 2H, NH2), 4.59 (s, 1H, CH), 2.67 (s, 3H CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 163.9, 152.0, 144.5, 138.0, 134.6, 134.3, 132.4, 131.8, 130.2, 129.6, 128.7, 128.5, 121.4, 110.0, 60.8, 37.0, 23.9, 23.3; IR (KBr) ν: 3452, 3098, 2941, 2876, 2184, 1771, 1716, 1676, 1626, 1559, 1489, 1417, 1379, 1280, 1181, 1079, 980, 839, 758 cm−1; MS (m/z): HRMS (ESI) calcd for C21H15ClN4NaO2 ([M + Na]+): 413.0776. Found: 413.0779.
2-Amino-1,4-bis(4-chlorophenyl)-6-methyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1h). Yellow solid, 0.210 g, 95%, mp 248–250 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.56 (d, J = 8.8 Hz, 2H, ArH), 7.48 (d, J = 8.8 Hz, 2H, ArH), 7.45–7.41 (m, 4H, ArH), 5.84 (s, 2H, NH2), 4.65 (s, 1H, CH), 2.67 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.8, 163.9, 152.1, 143.5, 138.2, 134.7, 134.1, 132.4, 132.2, 131.8, 130.5, 130.2, 129.9, 129.6, 129.2, 128.6, 121.3, 110.3, 60.8, 36.6, 23.9, 23.3; IR (KBr) ν: 3412, 2947, 2882, 2187, 1894, 1772, 1708, 1562, 1486, 1384, 1284, 1084, 982, 842, 753 cm−1; MS (m/z): HRMS (ESI) calcd for C21H16Cl2N4NaO3 ([M + Na]+): 465.0492. Found: 465.0493.
2-Amino-1-(4-bromophenyl)-6-methyl-5,7-dioxo-4-phenyl-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1i). Yellow solid, 0.184 g, 85%, mp 244–246 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.70–7.68 (m, 2H, ArH), 7.42–7.34 (m, 6H, ArH), 7.28–7.25 (m, 1H, ArH), 5.79 (s, 2H, NH2), 4.59 (s, 1H, CH), 2.67 (s, 3H CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 163.9, 152.0, 144.5, 138.0, 134.7, 133.1, 132.7, 132.0, 129.3, 128.7, 128.6, 127.9, 123.4, 121.4, 110.0, 60.8, 37.1, 23.9, 23.3; IR (KBr) ν: 3452, 3093, 2939, 2873, 2183, 1771, 1715, 1674, 1625, 1558, 1487, 1417, 1378, 1282, 1181, 1068, 980, 856, 757 cm−1; MS (m/z): HRMS (ESI) calcd for C21H15BrN4NaO2 ([M + Na]+): 457.0271. Found: 457.0277.
2-Amino-1-(4-bromophenyl)-4-(4-chlorophenyl)-6-methyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carbonitrile (1j). Yellow solid, 0.176 g, 75%, mp 243–245 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.69 (d, J = 8.8 Hz, 2H, ArH), 7.43–7.40 (m, 6H, ArH), 5.84 (s, 2H, NH2), 4.65 (s, 1H, CH), 2.67 (s, 3H CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.8, 163.8, 152.1, 143.4, 138.2, 134.6, 133.1, 132.6, 132.2, 130.5, 129.9, 129.2, 128.6, 123.4, 121.3, 110.3, 60.4, 36.6, 23.9, 23.3; IR (KBr) ν: 3454, 3093, 2940, 2885, 2180, 1774, 1716, 1675, 1627, 1557, 1484, 1418, 1377, 1278, 1149, 1074, 984, 837, 790 cm−1; MS (m/z): HRMS (ESI) calcd for C21H14BrClN4NaO2 ([M + Na]+): 490.9881. Found: 490.9885.
Ethyl 2-amino-6-methyl-4-(3-nitrophenyl)-5,7-dioxo-1-(p-tolyl)-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxylate (1k). Yellow solid, 0.143 g, 62%, mp 216–218 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.14 (s, 1H, ArH), 8.09–8.07 (m, 1H, ArH), 7.86–7.84 (m, 1H, ArH), 7.64–7.61 (m, 1H, ArH), 7.37 (s, 4H, ArH), 4.97 (s, 1H, CH), 3.90–3.88 (m, 2H, CH), 2.67 (s, 3H CH3), 2.42 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 168.9, 163.8, 154.0, 149.0, 147.9, 139.7, 138.0, 135.1, 132.3, 130.6, 130.2, 129.7, 122.7, 121.9, 112.2, 77.8, 59.2, 35.7, 23.6, 21.3, 14.4; IR (KBr) ν: 3414, 2989, 1718, 1687, 1600, 1523, 1486, 1374, 1352, 1308, 1223, 1073, 1017, 975, 847, 700 cm−1; MS (m/z): HRMS (ESI) calcd for C24H22N4NaO6 ([M + Na]+): 485.1432. Found: 485.1440.
Ethyl 2-amino-1-(4-chlorophenyl)-6-methyl-4-(3-nitrophenyl)-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxylate (1l). Yellow solid, 0.125 g, 52%, mp 258–260 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.14–8.13 (m, 1H, ArH), 8.09–8.06 (m, 1H, ArH), 7.87 (d, J = 7.2 Hz, 1H, ArH), 7.65–7.62 (m, 3H, ArH), 7.60–7.54 (m, 2H, ArH), 7.34 (brs, 2H, NH2), 4.96 (s, 1H, CH), 3.89 (q, J = 6.8 Hz, 2H, CH), 2.67 (s, 3H CH3), 0.99 (t, J = 6.0 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 168.9, 163.9, 153.7, 148.9, 147.9, 137.7, 135.2, 134.3, 133.0, 132.4, 130.2, 123.6, 122.8, 121.9, 112.2, 77.8, 59.2, 35.7, 23.6, 14.4; IR (KBr) ν: 3437, 3093, 2985, 1774, 1718, 1686, 1653, 1595, 1496, 1441, 1379, 1345, 1316, 1221, 1099, 1070, 1019, 986, 923, 899, 847, 785 cm−1; MS (m/z): HRMS (ESI) calcd for C23H19BrN4NaO6 ([M + Na]+): 505.0885. Found: 505.0882.
Ethyl 2-amino-1-(4-bromophenyl)-6-methyl-4-(3-nitrophenyl)-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxylate (1m). Yellow solid, 0.205 g, 78%, mp 233–235 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.15–8.14 (m, 1H, ArH), 8.08–8.06 (m, 1H, ArH), 7.87 (d, J = 7.6 Hz, 1H, ArH), 7.78–7.75 (m, 2H, ArH), 7.62 (d, J = 8.0 Hz, 1H, ArH), 7.49–7.47 (m, 2H, ArH), 7.33 (brs, 2H, NH2), 4.96 (s, 1H, CH), 3.89 (q, J = 6.8 Hz, 2H, CH), 2.67 (s, 3H CH3), 0.99 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 169.0, 168.9, 163.9, 153.7, 148.9, 147.9, 137.7, 135.2, 134.3, 133.0, 132.4, 130.2, 123.6, 122.8, 121.9, 112.2, 77.8, 59.2, 35.7, 23.6, 14.4; IR (KBr) ν: 3437, 3093, 2985, 1774, 1718, 1686, 1653, 1595, 1496, 1441, 1379, 1345, 1316, 1221, 1099, 1070, 1019, 986, 923, 899, 847, 785 cm−1; MS (m/z): HRMS (ESI) calcd for C23H19BrN4NaO6 ([M + Na]+): 549.0380. Found: 549.0370.
2-Amino-1-benzyl-6-methyl-5,7-dioxo-4-phenyl-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxamide (1n). Yellow solid, 0.107 g, 55%, mp 194–196 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.06 (s, 2H, NH2), 7.36–7.34 (m, 2H, ArH), 7.31–7.28 (m, 3H, ArH), 7.13 (s, 5H, ArH), 6.37 (s, 2H, NH2), 5.56 (d, J = 16.4 Hz, 1H, CH), 5.23 (d, J = 16.4 Hz, 1H, CH), 4.84 (s, 1H, CH), 2.78 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 172.1, 169.1, 165.9, 152.4, 152.3, 145.5, 137.8, 137.6, 129.0, 128.4, 127.9, 127.8, 127.6, 126.7, 114.1, 81.6, 45.9, 34.7, 23.7; IR (KBr) ν: 3431, 3168, 1763, 1682, 1603, 1477, 1427, 1378, 1232, 1067, 983, 830, 743 cm−1; MS (m/z): HRMS (ESI) calcd for C22H20N4NaO3 ([M + Na]+): 411.1428. Found: 411.1436.
2-Amino-1-benzyl-4-(4-chlorophenyl)-6-methyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxamide (1o). Yellow solid, 0.144 g, 68%, mp 170–172 °C; 1H NMR (400 MHz, DMSO-d6) δ: 8.10 (s, 2H, NH2), 7.39–7.32 (m, 3H, ArH), 7.28–7.26 (m, 2H, ArH), 7.22–7.19 (m, 2H, ArH), 7.13–7.12 (m, 2H, ArH), 6.42 (s, 2H, NH2), 5.53 (d, J = 16.4 Hz, 1H, CH), 5.24 (d, J = 16.4 Hz, 1H, CH), 4.88 (s, 1H, CH), 2.78 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 172.0, 169.1, 165.8, 152.5, 144.5, 138.1, 137.5, 131.4, 129.8, 129.0, 128.3, 128.0, 127.8, 127.6, 113.6, 81.2, 46.0, 34.2, 23.8; IR (KBr) ν: 3499, 3442, 3140, 2944, 1763, 1682, 1677, 1608, 1479, 1424, 1381, 1232, 1172, 1088, 983, 841, 749 cm−1; MS (m/z): HRMS (ESI) calcd for C22H19ClN4NaO3 ([M + Na]+): 445.1038. Found: 445.1041.
2-Amino-4-(4-chlorophenyl)-6-methyl-5,7-dioxo-1-(p-tolyl)-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxamide (1p). Yellow solid, 0.179 g, 85%, mp 232–234 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.48–7.46 (m, 2H, ArH), 7.38–7.36 (m, 2H, ArH), 7.33–7.28 (m, 4H, ArH), 6.44 (s, 2H, NH2), 4.98 (s, 1H, CH), 2.65 (s, 3H CH3), 2.40 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6) δ: 171.9, 169.3, 164.0, 151.8, 145.2, 139.3, 137.5, 132.9, 131.5, 130.4, 130.0, 129.7, 128.6, 112.1, 79.8, 34.5, 23.5, 21.3; IR (KBr) ν: 3506, 3459, 3125, 1770, 1694, 1627, 1577, 1470, 1412, 1377, 1260, 1177, 1056, 982, 833, 748 cm−1; MS (m/z): HRMS (ESI) calcd for C22H19ClN4NaO3 ([M + Na]+): 445.1038. Found: 445.1050.
2-Amino-1-(4-bromophenyl)-4-(4-chlorophenyl)-6-methyl-5,7-dioxo-4,5,6,7-tetrahydro-1H-pyrrolo[3,4-b]pyridine-3-carboxamide (1q). Yellow solid, 0.199 g, 82%, mp 243–245 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.72–7.70 (m, 2H, ArH), 7.48–7.46 (m, 2H, ArH), 7.43–7.40 (m, 4H, ArH), 6.54 (s, 2H, NH2), 4.97 (s, 1H, CH), 2.66 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6) δ: 171.5, 168.8, 163.6, 151.1, 144.6, 136.7, 134.4, 132.3, 132.0, 131.0, 129.6, 128.1, 122.7, 111.8, 79.4, 34.1, 23.1; IR (KBr) ν: 3502, 3460, 3121, 1769, 1692, 1627, 1579, 1473, 1381, 1265, 1065, 984, 838, 750 cm−1; MS (m/z): HRMS (ESI) calcd for C21H16BrClN4NaO3 ([M + Na]+): 508.9987. Found: 508.9988.
2. General procedure for the synthesis of chain products 2a,2b and 4a,4b
A mixture of 3-arylamino-1-methyl-1H-pyrrole-2,5-dione (0.5 mmol), aromatic aldehyde (0.5 mmol) dimedone or 4-hydroxy-2H-chromen-2-one (0.5 mmol) and acetic acid (5.0 mL) in ethanol (10.0 mL) was stirred at room temperature for about eight hours. TLC monitor indicated that the reaction has finished. After removing the solvent by rotator evaporation at reduced pressure, the residue was subjected to preparative thin-layer chromatography with a mixture of light petroleum and ethyl acetate (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as developing agents to give the pure product for analysis.
1) as developing agents to give the pure product for analysis.
3-((4-Chlorophenyl)amino)-4-((2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1-methyl-1H-pyrrole-2,5-dione (2a). Yellow solid, 0.163 g, 66%; mp 152–154 °C; 1H NMR (600 MHz, CDCl3) δ: 8.93 (s, 1H, OH), 7.91 (s, 1H, NH), 7.26 (s, 2H, ArH), 7.08–7.05 (m, 4H, ArH), 6.86–6.84 (m, 2H, ArH), 5.51 (s, 1H, CH), 3.80 (s, 3H, OCH3), 3.01 (s, 3H, CH3), 2.39–2.30 (m, 4H, 2CH2), 1.15 (s, 3H, CH3), 1.11 (s, 3H, CH3); 13C NMR (150 MHz, CDCl3) δ: 199.1, 175.1, 173.1, 166.7, 158.2, 140.7, 137.3, 129.9, 129.8, 128.4, 128.2, 122.6, 114.0, 109.3, 58.4, 55.2, 50.2, 43.7, 31.3, 31.1, 29.5, 27.2, 23.8, 18.4; IR (KBr) ν: 3212, 3102, 3039, 2959, 1767, 1688, 1647, 1598, 1504, 1453, 1383, 1291, 1246, 1169, 1099, 1030, 964, 826, 750 cm−1; MS (m/z): HRMS (ESI) calcd for C27H27ClN2NaO5 ([M + Na]+): 517.1501. Found: 517.1511.
3-((4-Chlorophenyl)amino)-4-((2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(p-tolyl)methyl)-1-methyl-1H-pyrrole-2,5-dione (2b). Yellow solid, 0.167 g, 70%; mp 156–158 °C; 1H NMR (400 MHz, CDCl3) δ: 8.92 (s, 1H, OH), 7.91 (s, 1H, NH), 7.20–7.18 (m, 2H, ArH), 7.11 (d, J = 8.0 Hz, 2H, ArH), 7.06–7.03 (m, 4H, ArH), 5.52 (s, 1H, CH), 3.00 (s, 3H, CH3), 2.38–2.36 (m, 2H, CH2), 2.32 (s, 3H, CH3), 2.30–2.28 (m, 2H, CH2), 1.15 (s, 3H, CH3), 1.10 (s, 3H, CH3); 13C NMR (150 MHz, CDCl3) δ: 195.6, 168.3, 163.7, 151.6, 151.0, 146.8, 137.6, 136.0, 135.4, 130.2, 130.0, 128.9, 123.9, 113.98, 113.1, 50.0, 40.6, 34.4, 32.4, 29.2, 27.1, 23.5; IR (KBr) ν: 3246, 3103, 3032, 2957, 1764, 1695, 1646, 1600, 1501, 1450, 1388, 1244, 1155, 1095, 1011, 824, 750; IR (KBr) ν: 3246, 3103, 3032, 2957, 1764, 1695, 1646, 1600, 1501, 1450, 1388, 1244, 1155, 1095, 1011, 824, 750 cm−1; MS (m/z): HRMS (ESI) calcd for C27H27Cl2NaO4 ([M + Na]+): 501.1552; found: 501.1560.
3-((4-Hydroxy-2-oxo-2H-chromen-3-yl)(phenyl)methyl)-1-methyl-4-(p-tolylamino)-1H-pyrrole-2,5-dione (4a). Red solid, 0.144 g, 62%, mp 228–230 °C; 1H NMR (600 MHz, DMSO-d6) δ: 11.04 (s, 1H, OH), 7.77 (d, J = 7.8 Hz, 1H, ArH), 7.38–7.35 (m, 1H, ArH), 7.15–7.14 (m, 2H, ArH), 7.11–7.08 (m, 4H, ArH), 7.01–6.98 (m, 1H, ArH), 6.95–6.94 (m, 2H, ArH), 6.83–6.82 (m, 2H, ArH), 5.77 (s, 1H, CH), 2.84 (s, 3H, CH3), 2.19 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6) δ: 176.7, 176.2, 171.4, 169.6, 157.4, 147.0, 146.2, 142.7, 135.0, 134.0, 133.0, 131.7, 131.2, 128.8, 128.7, 127.5, 126.0, 124.4, 119.5, 118.1, 102.5, 83.4, 83.2, 83.0, 37.9, 27.6, 24.6; IR (KBr) ν: 3068, 2933, 2855, 1764, 1684, 1639, 1512, 1451, 1386, 1282, 1239, 1159, 110, 1035, 912, 811, 759 cm−1; MS (m/z): HRMS (ESI) calcd for C28H22N2NaO5 ([M + Na]+): 489.1421; found: 489.1425.
3-((4-Hydroxy-2-oxo-2H-chromen-3-yl)(4-nitrophenyl)methyl)-1-methyl-4-(p-tolylamino)-1H-pyrrole-2,5-dione (4b). Red solid, 0.174 g, 68%, mp 242–244 °C; 1H NMR (600 MHz, DMSO-d6) δ: 10.94 (s, 1H, OH), 8.00 (d, J = 9.0 Hz, 2H, ArH), 7.75 (d, J = 7.2 Hz, 1H, ArH), 7.42–7.38 (m, 3H, ArH), 7.13–7.10 (m, 2H, ArH), 6.96–6.95 (m, 2H, ArH), 6.88–6.86 (m, 2H, ArH), 5.82 (s, 1H, CH), 2.86 (s, 3H, CH3), 2.20 (s, 3H, CH3); 13C NMR (150 MHz, DMSO-d6) δ: 170.3, 172.2, 167.2, 165.6, 153.7, 152.6, 145.4, 142.8, 138.4, 131.8, 130.6, 129.2, 128.5, 124.9, 123.4, 123.3, 122.6, 121.0, 115.9, 111.8, 110.0, 98.4, 55.5, 34.6, 23.9, 20.8, 19.0; IR (KBr) ν: 3188, 2921, 1762, 1697, 1643, 1601, 1514, 1447, 1388, 1344, 1243, 1198, 1158, 1112, 1045, 990, 907, 860, 811, 761 cm−1; MS (m/z): HRMS (ESI) calcd for C28H21N3NaO7 ([M + Na]+): 534.1272; found: 534.1268.
3. General procedure for the synthesis of functionalized pyrrolo[3,4-b]quinoline 3a–3j
A mixture of 3-arylamino-1-methyl-1H-pyrrole-2,5-dione (0.5 mmol), aromatic aldehyde (0.5 mmol) and dimedone (cyclohexane-1,3-dione) (0.5 mmol) in acetic acid (10.0 mL) was refluxed for about ten hours. TLC monitor indicated that the reaction has finished. After removing the solvent by rotator evaporation at reduced pressure, the residue was subjected to preparative thin-layer chromatography with a mixture of light petroleum and ethyl acetate (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as developing agents to give the pure product for analysis.
1) as developing agents to give the pure product for analysis.
2-Methyl-9-phenyl-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3a). Yellow solid, 0.149 g, 74%, mp 230–232 °C; 1H NMR (600 MHz, CDCl3) δ: 7.41–7.40 (m, 2H, ArH), 7.33–7.31 (m, 4H, ArH), 7.21–7.16 (m, 3H, ArH), 5.20 (s, 1H, CH), 2.80 (s, 3H, CH3), 2.45 (s, 3H, CH3), 2.42–2.38 (m, 1H, CH), 2.33–2.30 (m, 1H, CH), 2.28–2.26 (m, 2H, CH2), 1.96–1.93 (m, 2H, CH2), 1.88–1.82 (m, 1H, CH); 13C NMR (150 MHz, CDCl3) δ: 196.0, 168.9, 164.4, 153.4, 144.5, 139.8, 137.3, 134.8, 130.3, 128.6, 128.5, 127.8, 115.1, 114.6, 36.8, 33.6, 27.2, 23.3, 21.4, 21.0; IR (KBr) ν: 3071, 3030, 2950, 2884, 1763, 1710, 1668, 1569, 1508, 1443, 1368, 1252, 1160, 1106, 1060, 1017, 975, 831, 776, 740, 703 cm−1; MS (m/z): HRMS (ESI) calcd for C25H22N2NaO3 ([M + Na]+): 425.1523; found: 425.1533.
2,7,7-Trimethyl-4,9-di-p-tolyl-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3b). Yellow solid, 0.176 g, 80%, mp 216–218 °C; 1H NMR (400 MHz, CDCl3) δ: 7.34–7.28 (m, 4H, ArH), 7.16–7.12 (m, 4H, ArH), 5.10 (s, 1H, CH), 2.70 (s, 3H, CH3), 2.47 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.26–2.22 (m, 1H, CH), 2.20–2.27 (m, 1H, CH), 2.15–2.14 (m, 1H, CH), 2.08–2.04 (m, 1H, CH), 1.00 (s, 3H, CH3), 0.92 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 195.8, 168.9, 164.5, 151.3, 141.7, 139.7, 136.5, 134.8, 130.4, 129.3, 127.8, 121.9, 115.2, 113.8, 50.3, 40.5, 33.5, 32.3, 29.4, 27.1, 23.3, 21.4, 21.1; IR (KBr) ν: 3268, 3029, 2953, 2923, 2874, 1761, 1693, 1651, 1604, 1518, 1448, 1395, 1334, 1240, 1154, 994, 821, 749 cm−1; MS (m/z): HRMS (ESI) calcd for C28H28N2NaO3 ([M + Na]+): 463.1992; found: 463.1995.
9-(4-(tert-Butyl)phenyl)-2,7,7-trimethyl-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3c). Yellow solid, 0.159 g, 66%, mp 182–184 °C; 1H NMR (400 MHz, CDCl3) δ: 7.34–7.31 (m, 6H, ArH), 7.17–7.15 (m, 2H, ArH), 5.12 (s, 1H, CH), 2.80 (s, 3H, CH3), 2.47 (s, 3H, CH3), 2.23–2.10 (m, 2H, CH2), 2.17–2.14 (m, 1H, CH), 2.08–2.04 (m, 1H, CH), 1.27 (s, 9H, 3CH3), 1.00 (s, 3H, CH3), 0.94 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 195.9, 169.0, 164.5, 151.5, 149.5, 141.5, 139.7, 137.3, 134.8, 130.4, 127.4, 125.6, 118.8, 115.4, 113.7, 50.3, 40.6, 34.4, 33.3, 32.3, 29.4, 27.7, 23.3, 21.4; IR (KBr) ν: 3043, 2953, 1768, 1712, 1664, 1577, 1508, 1445, 1365, 1320, 1252, 1150, 1111, 1028, 978, 928, 836, 744 cm−1; MS (m/z): HRMS (ESI) calcd for C31H34N2NaO3 ([M + Na]+): 505.2462; found: 505.2475.
2,7,7-Trimethyl-9-(4-nitrophenyl)-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3d). Yellow solid, 0.175 g, 74%, mp 224–226 °C; 1H NMR (400 MHz, CDCl3) δ: 8.20 (J = 8.8 Hz, 2H, ArH), 7.58 (d, J = 8.8 Hz, 2H, ArH), 7.35 (d, J = 8.8 Hz, 2H, ArH), 7.16 (d, J = 8.8 Hz, 2H, ArH), 5.26 (s, 1H, CH), 2.61 (s, 3H, CH3), 2.48 (s, 3H, CH3), 2.77–2.20 (m, 2H, CH), 2.16–2.07 (m, 2H, CH2), 1.00 (s, 3H, CH3), 0.92 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 195.7, 168.5, 168.8, 152.3, 151.4, 146.8, 140.1, 138.0, 134.2, 130.5, 128.8, 123.9, 113.2, 112.8, 50.0, 40.5, 34.4, 32.3, 29.3, 27.1, 23.4, 21.4; IR (KBr) ν: 3443, 2958, 1769, 1713, 1668, 1574, 1516, 1446, 1353, 1251, 1154, 1103, 1035, 977, 862, 828, 742, 700 cm−1; MS (m/z): HRMS (ESI) calcd for C27H25N3NaO5 ([M + Na]+): 494.1686. Found: 494.1689.
2-Methyl-9-(4-nitrophenyl)-4-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3e). Yellow solid, 0.173 g, 78%, mp 216–218 °C; 1H NMR (600 MHz, CDCl3) δ: 8.19 (d, J = 6.0 Hz, 2H, ArH), 7.57 (d, J = 6.0 Hz, 2H, ArH), 7.34–7.33 (m, 2H, ArH), 7.18–7.16 (m, 2H, ArH), 5.30 (s, 1H, CH), 2.81 (s, 3H, CH3), 2.46 (s, 3H, CH3), 2.42–2.38 (m, 1H, CH), 2.35–2.32 (m, 1H, CH), 2.30–2.28 (m, 2H, CH2), 2.00–1.96 (m, 1H, CH), 1.86–1.84 (m, 1H, CH); 13C NMR (150 MHz, CDCl3) δ: 195.8, 168.6, 163.9, 154.2, 151.4, 146.9, 140.1, 137.8, 134.4, 130.5, 128.9, 128.4, 124.0, 113.7, 113.5, 36.6, 34.3, 27.3, 23.5, 21.4, 21.0; IR (KBr) ν: 3071, 2931, 2880, 1925, 1769, 1713, 1671, 1604, 1567, 1517, 1442, 1355, 1272, 1247, 1162, 1105, 1060, 1016, 973, 905, 826, 775, 740, 704 cm−1; MS (m/z): HRMS (ESI) calcd for C25H21N3NaO5 ([M + Na]+): 466.1373; found: 466.1374.
2-Methyl-9-(4-methoxyphenyl)-4-phenyl-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3f). Yellow solid, 0.145 g, 70%, mp 216–218 °C; 1H NMR (600 MHz, CDCl3) δ: 7.40–7.39 (m, 2H, ArH), 7.32–7.31 (m, 2H, ArH), 7.20–7.19 (m, 3H, ArH), 7.10–6.99 (m, 2H, ArH), 5.20 (s, 1H, CH), 3.87 (s, 3H, OCH3), 2.80 (s, 3H, CH3), 2.42–2.39 (m, 1H, CH), 2.32–2.30 (m, 1H, CH), 2.28–2.26 (m, 2H, CH2), 1.98–1.94 (m, 1H, CH), 1.88–1.84 (m, 1H, CH); 13C NMR (150 MHz, CDCl3) δ: 195.9, 168.9, 164.4, 160.2, 153.7, 144.5, 137.4, 129.9, 128.6, 127.8, 126.6, 115.0, 114.7, 114.6, 35.6, 36.8, 33.7, 27.2, 23.3, 21.1; IR (KBr) ν: 3072, 3027, 2946, 2892, 2829, 1887, 1764, 1711, 1668, 1571, 1507, 1446, 1369, 1286, 1244, 1159, 1105, 1068, 1025, 975, 917, 841, 777, 741, 701 cm−1; MS (m/z): HRMS (ESI) calcd for C25H22N2NaO4 ([M + Na]+): 437.1472; found: 437.1484.
4-(4-Methoxyphenyl)-2,7,7-trimethyl-9-(4-nitrophenyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trion (3g). Yellow solid, 0.175 g, 72%, mp 208–210 °C; 1H NMR (400 MHz, CDCl3) δ: 8.20 (d, J = 8.0 Hz, 2H, ArH), 7.57 (d, J = 8.0 Hz, 2H, ArH), 7.20–7.17 (m, 2H, ArH), 7.05–7.03 (m, 2H, ArH), 5.26 (s, 1H, CH), 3.90 (s, 3H, OCH3), 2.82 (s, 3H, CH3), 2.28–2.20 (m, 2H, CH2), 2.16–2.08 (m, 2H, CH2), 1.01 (s, 3H, CH3), 0.92 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 191.0, 163.8, 159.2, 155.5, 147.9, 146.6, 142.0, 133.2, 124.5, 124.1, 119.2, 110.2, 108.5, 108.0, 50.8, 45.3, 35.7, 29.7, 27.6, 18.7; IR (KBr) ν: 3068, 2950, 1767, 1710, 1668, 1575, 1516, 1449, 1352, 1303, 1247, 1156, 1101, 1025, 974, 835, 746, 703 cm−1; MS (m/z): HRMS (ESI) calcd for C27H25N3NaO6 ([M + Na]+): 510.1636. Found: 510.1633.
4-(4-Chlorophenyl)-2-methyl-9-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3h). Yellow solid, 0.156 g, 72%, mp 240–242 °C; 1H NMR (600 MHz, CDCl3) δ: 7.50–7.48 (m, 2H, ArH), 7.28–7.26 (m, 2H, ArH), 7.25–7.24 (m, 2H, ArH), 7.13–7.12 (m, 2H, ArH), 5.15 (s, 1H, CH), 2.80 (s, 3H, CH3), 2.41–2.38 (m, 1H, CH), 2.33–2.27 (m, 1H, CH), 2.25–2.24 (m, 2H, CH2), 1.98–1.94 (m, 1H, CH), 1.88–1.84 (m, 1H, CH); 13C NMR (150 MHz, CDCl3) δ: 195.8, 168.7, 164.4, 152.5, 141.4, 136.8, 136.7, 135.9, 135.7, 130.3, 129.9, 129.4, 127.7, 115.7, 115.0, 36.8, 33.2, 27.3, 23.4, 21.1, 21.0; IR (KBr) ν: 3088, 2953, 2882, 1912, 1763, 1709, 1668, 1568, 1485, 1368, 1263, 1155, 1095, 1057, 1014, 973, 844, 776, 735 cm−1; MS (m/z): HRMS (ESI) calcd for C25H21ClN2NaO3 ([M + Na]+): 455.1133; found: 455.1132.
4-(4-Chlorophenyl)-2,7,7-trimethyl-9-(4-nitrophenyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3i). Yellow solid, 0.184 g, 75%, mp 226–228 °C; 1H NMR (400 MHz, CDCl3) δ: 8.21 (s, 1H, ArH), 8.19 (s, 1H, ArH), 7.58 (s, 1H, ArH), 7.56 (d, J = 8.8 Hz, 2H, ArH), 7.52 (s, 1H, ArH), 7.24–7.22 (m, 2H, ArH), 5.25 (s, 1H, CH), 2.82 (s, 3H, CH3), 2.28–2.24 (s, 1H, CH), 2.21–2.18 (m, 1H, CH), 2.17–2.14 (m, 1H, CH), 2.08–2.04 (m, 1H, CH), 1.02 (s, 3H, CH3), 0.93 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 195.6, 168.3, 163.8, 151.6, 146.8, 137.6, 136.0, 135.4, 130.2, 130.0, 128.9, 124.0, 113.8, 113.1, 50.0, 40.6, 34.4, 32.4, 29.2, 27.1, 23.5; IR (KBr) ν: 3438, 2957, 1771, 1715, 1669, 1570, 1517, 1443, 1356, 1254, 1155, 1096, 1025, 976, 835, 743, 703 cm−1; MS (m/z): HRMS (ESI) calcd for C26H22CN3NaO5 ([M + Na]+): 514.1140; found: 514.1139.
4-(4-Bromophenyl)-2-methyl-9-(p-tolyl)-6,7,8,9-tetrahydro-1H-pyrrolo[3,4-b]quinoline-1,3,5(2H,4H)-trione (3j). Yellow solid, 0.162 g, 68%, mp 238–240 °C; 1H NMR (600 MHz, CDCl3) δ: 7.65 (d, J = 8.4 Hz, 2H, ArH), 7.28–7.26 (m, 2H, ArH), 7.18 (d, J = 8.4 Hz, 2H, ArH), 7.13–7.12 (m, 2H, ArH), 5.15 (s, 1H, CH), 2.80 (s, 3H, CH3), 2.42–2.38 (m, 1H, CH), 2.33–2.28 (m, 1H, CH), 2.30 (s, 3H, CH3), 2.25–2.23 (m, 2H, CH2), 1.98–1.94 (m, 1H, CH), 1.90–1.84 (m, 1H, CH); 13C NMR (150 MHz, Hz, CDCl3) δ: 195.8, 168.7, 164.4, 152.4, 141.4, 136.7, 136.5, 132.9, 13.6, 129.4, 127.7, 123.8, 115.7, 115.1, 36.8, 33.2, 30.9, 27.3, 23.4, 21.1, 21.0, 18.5; IR (KBr) ν: 3068, 3018, 2950, 1909, 1801, 1764, 1709, 1669, 1639, 1568, 1486, 1474, 1368, 1260, 1157, 1104, 1059, 1014, 976, 915, 831, 775, 740 cm−1; MS (m/z): HRMS (ESI) calcd for C25H21BrNNaO3 ([M + Na]+): 499.0628; found: 499.063.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21172189, 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank the Analysis and Test Center of Yangzhou University providing instruments for analysis.
References
-
(a) S. S. Hegde, M. W. Vetting, S. L. Roderick, L. A. Mitchenall, A. Maxwell, H. E. Takiff and J. S. Blanchard, Science, 2005, 308, 1480–1483 CrossRef CAS PubMed;
(b) B. D. Bax, P. F. Chan, D. S. Eggleston, A. Fosberry, D. R. Gentry, F. Gorrec, I. Gior-dano, M. M. Hann, A. Hennessy, M. Hibbs, J. Huang, E. Jones, J. Jones, K. K. Brown, C. J. Lewis, E. W. May, M. R. Saunders, O. Singh, C. E. Spitzfaden, C. Shen, A. Shillings, A. J. Theobald, A. Wohlkonig, N. D. Pearson and M. N. Gwynn, Nature, 2010, 466, 935–940 CrossRef PubMed.
-
(a) A. Carbone, M. Pennati, B. Parrino, A. Lopergolo, P. Barraja, A. Montalbano, V. Spano, S. Sbarra, V. Doldi, M. De Cesare, G. Cirrincione, P. Diana and N. Zaffaroni, J. Med. Chem., 2013, 56, 7060–7072 CrossRef CAS PubMed;
(b) X. Zhao, W. Huang, Y. Wang, M. Xin, Q. Jin, J. Cai, F. Tang, Y. Zhao and H. Xiang, Bioorg. Med. Chem., 2015, 23, 4344–4353 CrossRef CAS PubMed;
(c) H. Yamagishi, S. Shirakami, Y. Nakajimi, A. Tanaka, F. Takahasi, H. Hamaguchi, K. Hatanaka, A. Moritomo, M. Inami, Y. Higashi and T. Inoue, Bioorg. Med. Chem., 2015, 23, 4846–4859 CrossRef CAS PubMed.
-
(a) A. M. Abdel-Aziz, Eur. J. Med. Chem., 2007, 42, 614–626 CrossRef CAS PubMed;
(b) G. K. Freschauf, F. Karimi-Busheri, A. Ulaczyk-Lesanko, T. R. Mereniuk, A. Ahrens, J. M. Koshy, A. Rasouli-Nia, P. Pasarj, C. F. B. Holmes and F. Rininsland, Cancer Res., 2009, 69, 7739–7746 CrossRef CAS PubMed;
(c) G. K. Freschauf, R. S. Mani, T. R. Mereniuk, M. Fanta, C. A. Virgen, G. L. Dianov, J.-M. Grassot, D. G. Hall and M. Weinfeld, J. Biol. Chem., 2010, 285, 2351–2360 CrossRef CAS PubMed;
(d) R. van der Westhuyzen, S. Winks, C. R. Wilson, G. A. Boyle, R. K. Gessner, C. Soares de Melo, D. Taylor, C. de Kock, M. Njoroge, C. Brunschwig, N. Lawrence, S. P. S. Rao, F. Sirgel, P. van Helden, R. Seldon, A. Moosa, D. F. Warner, L. Arista, U. H. Manjunatha, P. W. Smith, L. J. Street and K. Chibale, J. Med. Chem., 2015, 58, 9371–9381 CrossRef CAS PubMed.
-
(a) R. Madhav, Synthesis, 1973, 609–610 CrossRef CAS;
(b) C. K. Sha, C. P. Tsou, C. Y. Tsai, J. M. Liu, R. S. Lee, Y. C. Li, F. Y. Tsai, S. J. Way and J. J. Young, J. Chin. Chem. Soc., 1992, 39, 635–639 CrossRef CAS;
(c) K. Estieu, R. Paugam, J. Ollivier, J. Salauen, F. M. Cordero, A. Goti and A. Brandi, J. Org. Chem., 1997, 62, 8276–8277 CrossRef CAS PubMed.
-
(a) X. W. Sun, P. Janvier, G. Zhao, H. Bienayme and J. P. Zhu, Org. Lett., 2001, 3, 877–880 CrossRef CAS PubMed;
(b) D. Blanco-Ania, P. H. H. Hermkens, L. A. J. M. Sliedregt, H. W. Scheeren and F. P. J. T. Rutjes, J. Comb. Chem., 2009, 11, 539–546 CrossRef CAS PubMed.
-
(a) M. Chiurato, R. Boulahjar, S. Routier, Y. Troin and G. Guillaumet, Tetrahedron, 2010, 66, 4647–4653 CrossRef CAS;
(b) M. I. Escudero, L. D. Kremenchuzky, I. A. Perillo, H. Cerecetto and M. M. Blanco, Synthesis, 2011, 571–576 CAS;
(c) A. Plas, I. Abrunhosa-Thomas, P. Chalard and Y. Troin, Tetrahedron Lett., 2011, 52, 6873–6876 CrossRef CAS.
-
(a) G. X. Li, L. Wu, Q. Q. Fu, Z. Tang and X. M. Zhang, Sci. China: Chem., 2013, 56, 307–311 CrossRef CAS;
(b) Y. Y. Li, A. M. Wang, Y. Q. Shen and P. F. Zhang, J. Mol. Catal. B: Enzym., 2014, 110, 178–183 CrossRef CAS;
(c) M. M. Mashaly, S. R. El-Gogary and T. R. Kosbar, J. Heterocycl. Chem., 2014, 51(4), 1078–1085 CrossRef CAS.
-
(a) J. Sun, E. Y. Xia, Q. Wu and C. G. Yan, Org. Lett., 2010, 12, 3678–3681 CrossRef CAS PubMed;
(b) J. Sun, E. Y. Xia, Q. Wu and C. G. Yan, ACS Comb. Sci., 2011, 13, 421–426 CrossRef CAS PubMed;
(c) J. Sun, Y. Sun, E. Y. Xia and C. G. Yan, ACS Comb. Sci., 2011, 13, 436–441 CrossRef CAS PubMed;
(d) J. Sun, Q. Wu, E. Y. Xia and C. G. Yan, Eur. J. Org. Chem., 2011, 2981–2986 CrossRef CAS;
(e) J. Sun, Y. Sun, H. Gao and C. G. Yan, Eur. J. Org. Chem., 2011, 6952–6956 CrossRef CAS.
-
(a) A. Ziyaei-Halimehjani and M. R. Saidi, Tetrahedron Lett., 2008, 49, 1244–1248 CrossRef CAS;
(b) T. Glotova, M. Dvorko, I. Ushakov, N. Chipanina, O. Kazheva, A. Chekhlov, O. Dyachenko, N. Gusarova and B. Trofimov, Tetrahedron, 2009, 65, 9814–9818 CrossRef CAS;
(c) X. Li, J. Y. Wang, W. Yu and L. M. Wu, Tetrahedron, 2009, 65, 1140–1146 CrossRef CAS;
(d) I. Yavari, M. J. Bayat, M. Sirouspour and S. Souri, Tetrahedron, 2010, 66, 7995–7999 CrossRef CAS.
-
(a) A. Z. Elassara and A. A. El-Khair, Tetrahedron, 2003, 59, 8463–8480 CrossRef;
(b) M. Q. Fan, Z. Y. Yan, W. M. Liu and Y. M. Liang, J. Org. Chem., 2005, 70, 8204–8207 CrossRef CAS PubMed;
(c) T. B. Nguyen, A. Martel, R. Dhal and G. Dujardin, Org. Lett., 2008, 10, 4493–4496 CrossRef CAS PubMed;
(d) Q. H. Zhu, H. F. Jiang, J. H. Li, M. Zhang, X. J. Wang and C. R. Qi, Tetrahedron, 2010, 66, 9721–9728 CrossRef.
-
(a) L. Lu, J. M. Wei, J. Chen, J. P. Zhang, H. M. Deng, M. Shao, H. Zhang and W. G. Cao, Tetrahedron, 2009, 65, 9152–9156 CrossRef CAS;
(b) P. Singh, P. Sharma and K. Bisetty, Tetrahedron, 2009, 65, 8478–8485 CrossRef CAS;
(c) J. Bezenšek, T. Koleša, U. Grošelj, J. Wagger, K. Stare, A. Meden, J. Svete and B. Stanovnik, Tetrahedron Lett., 2010, 51, 3392–3397 CrossRef;
(d) M. B. Teimouri and T. Abbasi, Tetrahedron, 2010, 66, 3795–3800 CrossRef CAS.
-
(a) B. Das, G. Chinna Reddy, P. Balasubramanyam and V. Aneyulu, Synthesis, 2010, 1625–1628 CrossRef CAS;
(b) L. Nagarapu, R. Mallepalli, L. Yeramanchi and R. Bantu, Tetrahedron Lett., 2011, 52, 3401–3404 CrossRef CAS;
(c) K. Ramesh, N. S. Murthy, K. Karnakar and Y. Nageswar, Tetrahedron Lett., 2011, 52, 3937–3941 CrossRef CAS;
(d) E. Ghabraie, S. Balalaie, M. Bararjanian, H. R. Bijanzadeh and F. Rominger, Tetrahedron, 2011, 67, 5415–5420 CrossRef CAS.
-
(a) G. W. Yin, Y. X. Zhu, N. N. Wang, P. Lu and Y. G. Wang, Tetrahedron, 2013, 69, 8353–8359 CrossRef CAS;
(b) C. P. Chen, H. B. Wang, Y. Q. Wang, Q. H. Zhu, Y. Xie and S. W. Liu, Tetrahedron, 2014, 70, 4379–4385 CrossRef;
(c) J. T. Bowler, C. R. Clausen, D. J. Blackburn and W. M. Wu, Tetrahedron Lett., 2014, 55, 6465–6466 CrossRef CAS PubMed;
(d) C. Wang, Y. H. Jiang and C. G. Yan, Mol. Diversity, 2014, 18, 809–820 CrossRef CAS PubMed.
-
(a) M. Augustin and J. Faust, Z. Chem., 1975, 15, 144–145 CrossRef CAS;
(b) M. Augustin, et al., Tetrahedron, 1984, 40, 3499–3502 CrossRef CAS;
(c) M. Augustin and M. Koehler, J. Prakt. Chem., 1984, 326, 401–406 CrossRef CAS;
(d) D. R. Maulding, J. Heterocycl. Chem., 1988, 25, 1777–1779 CrossRef CAS;
(e) M. M. Mashaly, S. R. El-Gogary and T. R. Kosbar, J. Heterocycl. Chem., 2014, 51, 1078–1085 CrossRef CAS.
-
(a) J. Sun, Y. Sun, H. Gong, Y. J. Xie and C. G. Yan, Org. Lett., 2012, 14, 5172–5175 CrossRef CAS PubMed;
(b) Y. Han, Y. Sun, J. Sun and C. G. Yan, Tetrahedron, 2012, 68, 8256–8260 CrossRef CAS;
(c) J. Sun, Y. Sun, H. Gao and C. Yan, Eur. J. Org. Chem., 2012, 1976–1983 CrossRef CAS;
(d) L. Wu, J. Sun and C. G. Yan, Org. Biomol. Chem., 2012, 10, 9452–9463 RSC;
(e) Li. Hui, H. Y. Li and C. G. Yan, Eur. J. Org. Chem., 2012, 3157–3164 CrossRef CAS.
-
(a) H. Gao, J. Sun and C. G. Yan, Tetrahedron, 2013, 69, 589–594 CrossRef CAS;
(b) J. Sun, H. Gong and C. G. Yan, Tetrahedron, 2013, 69, 10235–10244 CrossRef CAS;
(c) Q. Fu and C. G. Yan, Tetrahedron, 2013, 69, 5841–5849 CrossRef CAS;
(d) H. Gong, J. Sun and C. G. Yan, Synthesis, 2014, 46, 489–495 CrossRef;
(e) H. Gao, J. Sun and C. G. Yan, J. Org. Chem., 2014, 79, 4131–4136 CrossRef CAS PubMed;
(f) Y. Han, Y. J. Sheng and C. G. Yan, Org. Lett., 2014, 16, 2654–2657 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra for all new compounds. CCDC 1a (CCDC 1451318), 1p (CCDC 1451319), 2b (CCDC 1451320), 3a (CCDC 1451321), 3g (CCDC 1451322), and 3j (CCDC 1451323). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra03165j |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as developing agents to give the pure product for analysis.
1) as developing agents to give the pure product for analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as developing agents to give the pure product for analysis.
1) as developing agents to give the pure product for analysis.








