Two new magnetic nanocomposites of graphene and 12-tungestophosphoric acid: characterization and comparison of the catalytic properties in the green synthesis of 1,8-dioxo-octahydroxanthenes
E. Rafiee*ab and
M. Khodayaria
aDepartment of Inorganic Chemistry, Faculty of Chemistry, Razi University, Kermanshah, 67149, Iran. E-mail: ezzat_rafiee@yahoo.com; e.rafiei@razi.ac.ir; Fax: +98 831 4274559; Tel: +98 831 4274559
bInstitute of Nano Science and Nano Technology, Razi University, Kermanshah, 67149, Iran
First published on 7th April 2016
Abstract
Two well dispersed H3PW12O40 immobilized to magnetite graphene oxide (Fe3O4/GO/PW) and magnetite graphene aerogel (Fe3O4/GA/PW) nanocomposites were synthesized, via coprecipitation and coprecipitation-solvothermal methods, respectively. The catalytic activity, leaching and recyclability of the two prepared catalysts were compared in the synthesis of 1,8-dioxo-octahydroxanthenes (DOX) in water. Fe3O4/GO/PW showed higher activity, and lower leaching followed by a higher reusing number compared with Fe3O4/GA/PW. The as-prepared Fe3O4/GO/PW nanocomposite was fully characterized by transmission electron microscopy, scanning electron microscopy, a vibrating sample magnetometer, energy dispersive X-ray spectroscopy, X-ray diffraction, laser particle size analyzer, Fourier transform infrared spectroscopy, Brunauer–Emmett–Teller (BET) and inductively coupled plasma atomic emission spectroscopy (ICP-AES). The excellent conversions show that the catalyst has been used as a highly effective catalyst for the synthesis of DOX. The novelty of this catalyst is the low leaching of PW in aqueous media and reusability of the catalyst for at least four times with an appreciable loss of its catalytic activity.
Introduction
It is generally approved that there is a growing need for more environmentally acceptable processes in the chemical industry. This trend towards what has become known as ‘Green Chemistry’1,2 or ‘Sustainable Technology’ necessitates a paradigm shift from traditional concepts of process efficiency to one that assigns economic value to eliminating waste at source and avoiding the use of toxic or hazardous substances. The attention to the process parameters in the synthesis of stable magnetic nanoparticles (MNPs), to get a controlled product, is of particular importance in view of the utilization of their properties3–5 in various fields. They can be used in catalytic, environmental, biological, biomedical and electronical applications. Much attention has been addressed to the preparation of MNPs.3–5 Moreover different strategies have been developed to preserve their stability against agglomeration or precipitation and oxidation phenomena including polymers,6–8 silica,9,10 precious metals11,12 and carbon by coating of some compounds.13–21 The carbon covering has many advantages over other coatings due to its feasibility to be easily functionalized. The porous carbons, comprised of one-dimensional (1D) carbon nanotubes (CNTs) or two-dimensional (2D) graphenes have been used in a wide variety of fields, including energy storage, sensors, catalysis and environmental science and engineering.22–33 Furthermore, carbon-based catalysts have been proposed as low cost renewable “green catalysts” able to be prepared from either biomass or from household waste. Compared with a CNT monolith, graphene-based metamaterials, one atom-thick two dimensional layers of sp2 bonded carbon, possesses several unique properties, including highly thermal conductivity, fracture strength, large specific area and extraordinary electronic transport properties.34,35 Recently, the combination of various heteropoly acids (HPAs) with inorganic supports as nanocatalysts have been successfully developed and evaluated in a number of important industrial reactions.36–40 Supported HPA catalysts are important for various applications because of environmental and economic considerations. They also have excellent activity and present a greater number of surface acid sites than their bulk components.41,42 Moreover they have been pointed lately as versatile green catalysts for a variety of organic reactions.43,44 Immobilization of HPAs in the magnetically recoverable nanoparticle supports will provide facile production process and offer an opportunity for their applications in the chemical or pharmaceutical industries. In this study, two different kind of materials, namely magnetite graphene oxide (Fe3O4/GO) and magnetite graphene aerogel (Fe3O4/GA) were firstly prepared as supports for the immobilization of HPAs. An easy controlled one-step chemical coprecipitation method was used to introduce 2D Fe3O4/GO, meanwhile 3D Fe3O4/GA was prepared by combination of a modified hydrothermal reduction process and chemical coprecipitation methods. Afterwards, PW was immobilized on magnetic supports, Fe3O4/GO and Fe3O4/GA, through chemical coprecipitation method to produce Fe3O4/GO/PW and Fe3O4/GA/PW. These are novel heterogeneous catalytic systems that possesses both a high separation efficiency and a relatively high surface area to produce maximize catalyst loading and activity. The effects of these two supports on the catalytic activity, leaching, and recyclability were investigated. Based on the obtained results, Fe3O4/GO/PW was used for the syntheses of biologically useful building blocks, namely 1,8-dioxo-octahydroxanthenes (DOX) in water.45–53Experimental
Materials and methods
FeCl3·6H2O (99%) and FeSO4·7H2O (99%), NaOH (98%), HCl (37%), graphite, PW (>99), other reagents and solvents used in this work were obtained from Merck, Aldrich or Fluka without further purification. Transmission electron microscopy (TEM) was obtained using a TEM microscope (Jeol JEM-2100 with an accelerating voltage of 200 kV). Scanning electron microscopy (SEM) has been performed using an AIS2300C microscope with scanning range from 0 to 20 keV. Energy-dispersive X-ray (EDX) measurements were made with an IXRF model 550i attached to SEM. SEM/EDX samples were prepared by coating of solid particles into a conductive layer. The size distribution of the samples was obtained using a laser particle size analyzer (HPPS5001, Malvern, UK). X-ray diffraction (XRD) patterns were obtained on an Inel French, EQUINOX 3000 model X-ray diffractometer using Cu-Kα radiation. Fourier transform infrared (FTIR) spectra were recorded with KBr pellets using a FTIR spectrometer ALPHA. Brunauer–Emmett–Teller (BET) surface areas and pore volumes were measured on a sorptometer kelvin 1042 using nitrogen adsorption at 77 K. Magnetic properties were measured using a BHV-55, Riken, Japan vibrating sample magnetometer (VSM). The inductively coupled plasma atomic emission spectroscopy (ICP-AES) on a Spectro Ciros CCD spectrometer were used to leaching measurements. NMR spectra were recorded on a Bruker Avance 200 MHz NMR spectrometer with CDCl3 as solvent and TMS as internal standard. Thin layer chromatography on precoated silica gel fluorescent 254 nm (0.2 mm) on aluminum plates was used for monitoring the reactions.Preparation of the catalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 several times. Finally, the obtained Fe3O4/GA was vacuum-dried overnight at room temperature.
1 several times. Finally, the obtained Fe3O4/GA was vacuum-dried overnight at room temperature.General procedure for the synthesis of DOX
For the synthesis of DOX, to a mixture of an aldehyde (1.0 mmol), dimedone (2.0 mmol), water (4 mL) was added the catalyst (Fe3O4/GO/PW or Fe3O4/GA/PW, 0.02 g) in reflux condition. The progress of the reaction indicated by thin-layer chromatography (TLC). After completion of the reaction the mixture was cooled to room temperature and the solid (containing catalyst and product) was filtered and washed with water (10 mL). The product was dissolved in acetonitrile, and the catalyst was recovered from the product using an external magnet. The solvent was evaporated in a vacuum to obtain the crude product, which was purified by recrystallization from ethanol. The remaining catalyst was washed with diethyl ether, dried under vacuum and reused in a subsequent reaction.Large scale synthesis
Reaction of dimedone and benzaldehyde was selected for large-scale synthesis. The reaction of dimedone (20.0 mmol) with benzaldehyde (10.0 mmol), in the presence of Fe3O4/GO/PW (0.2 g) was done at 70 °C. Purification of the product and recovery of the catalyst are similar to above procedure.Leaching as well as heterogeneity test
To check the leaching of Fe3O4/GO/PW and Fe3O4/GA/PW, after completion of the reaction between dimedone and benzaldehyde, in the presence of catalyst in 4 mL water at 70 °C, the content of PW filtrates was evaluated quantitatively by ICP-AES. For rigorous proof of heterogeneity, a test was carried out by hot filtration of the catalyst from the reaction mixture after obtaining a conversion of about 50% was done. Then, the reaction was continued with the residual solution.Results and discussion
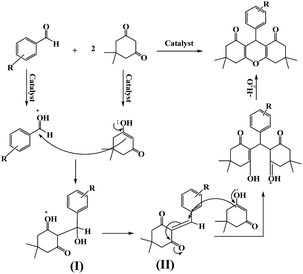
The synthetic process of Fe3O4/GO/PW and Fe3O4/GA/PW are indicated in Scheme 1 in two different ways. Graphite oxide was prepared by oxidation of graphite powder under harsh oxidizing conditions by Hummer's method and was converted to graphene oxide by ultrasonication. In pathway a, the Fe3O4/GO was prepared by chemical coprecipitation method of iron ions. Fe3+ and Fe2+ ions were captured by carboxylate anions on the surface of GO sheets. Then ammonia solution was gradually added to precipitate Fe3O4. Immobilization of Fe3O4/GO with a methanol solution of PW occurs via formation of hydrogen bonding between hydroxyl group on the surface of GO and PW molecule. Fe3O4/GA/PW was prepared by pathway b. Magnetic Fe3O4 NPs were prepared according to the literature using ferric chloride hexahydrate and ferrous chloride tetrahydrate upon addition of NH4OH through the chemical co-precipitation method. GO aqueous suspension was first mixed with reducing agent ethylenediamine and then freshly synthesized Fe3O4 nanoparticles. After hydrothermal treatment, GO was gradually self-assembled into a hydrogel with simultaneous deposition of Fe3O4 nanoparticles. The soft hydrogels, fabricated by the modified hydrothermal method, showed a very small volume shrinkage. Fe3O4/GA aerogel samples after drying were obtained. Afterwards, Fe3O4/GA was used as support for immobilization of PW.At the beginning, PW content of two different magnetic catalysts were compared with previously synthesized catalyst with carbon base, PW/SMNs (starch magnetic nanoparticles supported PW)57 with EDX spectrums. The EDX spectrums of the Fe3O4/GO/PW, Fe3O4/GA/PW and PW/SMNs are presented in Fig. 1a, b and c, respectively.
In Table 1 the molar ratio of carbon, oxygen, phosphor, iron and tungstate of the catalysts are illustrated. From these results, PW content of three different catalyst are similar. So, comparison of the catalytic activity and other factors for these catalysts are quite logical. Moreover, the EDX elemental mapping images spectrum of the Fe3O4/GO/PW, Fe3O4/GA/PW and PW/SMNs show uniform distribution of P and W (from PW) and C, O, Fe (from support).
| Element | Fe3O4/GO/PW | Fe3O4/GA/PW | PW/SMNs |
|---|---|---|---|
| C (%) | 11.145 | 10.321 | 11.895 |
| O (%) | 11.594 | 14.801 | 12.325 |
| P (%) | 2.730 | 2.671 | 2.92 |
| Fe (%) | 25.178 | 23.193 | 22.98 |
| W (%) | 49.354 | 49.014 | 49.88 |
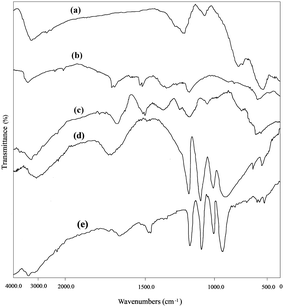
The FTIR spectra of GO, Fe3O4 nanoparticles, Fe3O4/GO, PW and Fe3O4/GO/PW are presented in Fig. 2a–e. FTIR spectrum of Fe3O4/GO58 (Fig. 2c) shown the presence of various functional groups like OH (3429 cm−1), COOH (1223 cm−1), C–O (824 cm−1) and C–O (1133 cm−1) related to graphene oxide. Also, Fe–O stretching vibration peak in the Fe3O4/GO was observed at 610 cm−1, in which for Fe3O4 (Fig. 2b) nanoparticles appear at 586 cm−1. PW Keggin structure is well known and comprised of a PO4 tetrahedron surrounded by four W3O13 groups designed by edge-sharing octahedra. These groups are linked to each other by corner-sharing oxygens.59 The described structure gives rise to four kindes of oxygen, being responsible for the fingerprint bands of the Keggin ion between 700 and 1200 cm−1. PW presents typical bands for absorptions at 1080 (P–O), 984 (W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 896 and 814 (W–O–W) cm−1 (Fig. 2d). In Fe3O4/GO/PW, the characteristic bands are at the same wavenumbers with Fe3O4/GO with a slight shift confirming the interaction to the support (Fig. 2e).
O), 896 and 814 (W–O–W) cm−1 (Fig. 2d). In Fe3O4/GO/PW, the characteristic bands are at the same wavenumbers with Fe3O4/GO with a slight shift confirming the interaction to the support (Fig. 2e).
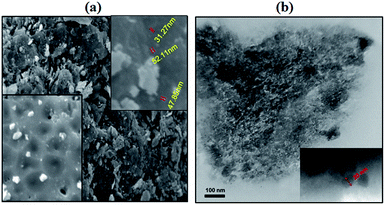
The SEM and TEM images of the synthesized Fe3O4/GO/PW are shown in Fig. 3a and b. From Fig. 3a it can be seen clearly that the iron nanoparticles and PW were successfully combined with the graphene sheets. Furthermore, it understand that GO nanosheets have rough surface which provide a large surface area to combination with Fe3O4 nanoparticles and PW coating. As shown in Fig. 3b, Fe3O4/GO/PW had well-defined composite structure composed of the graphene oxide sheets, Fe3O4 and PW nanoparticles. Closer examination reveals that the particles had an average diameter of about 15–20 nm.
Size distribution of the Fe3O4/GO and Fe3O4/GO/PW derived from a laser particle size analyzer, illustrated in Fig. 4a and b. Indicated that the mean diameter of them is 640 and 856 nm, respectively. As shown in the TEM image (Fig. 3b), aggregation of individual nano particles was occurred. Such aggregations cause the difference between particle size resultant from TEM analysis and the measurements made using the laser particle size analyzer.
The powder X-ray diffraction (XRD) patterns of Fe3O4/GO and Fe3O4/GO/PW are shown in Fig. 5. The diffraction peak in XRD at 10.1 was attributed to the planar structure of GO. Likewise, all the characteristic diffraction peaks of the pure crystal of Fe3O4 at 2θ of 30.35, 35.69, 43.38, 53.78 and 62.91 were present in the Fe3O4/GO which further indicated the successful synthesis of GO and Fe3O4/GO.60
When PW is impregnated on Fe3O4/GO, characteristic peaks assigned to PW are comparable to those for the Fe3O4/GO, which implies maintenance of their crystalline character. The presence of the peaks illustrates that there is no significant variation in the structure of the PW during the preparation which confirmed by FTIR spectroscopy.61
In order to investigate the porous structure and surface area of Fe3O4/GO/PW the N2 adsorption–desorption isotherm were conducted and shown in Fig. 6. The isotherm curve of Fe3O4/GO/PW is close to type IV with a weak hysteresis loop in the relative pressure, which indicated the presence of porous structure of Fe3O4/GO/PW composite. The measured BET surface area of the Fe3O4/GO/PW is 168.124 m2 g−1. In addition, the average pore diameter of the catalyst is 33.87 Å, calculated from desorption branch of the nitrogen isotherm with the BJH method. The corresponding BJH desorption cumulative pore volumes is 0.181 cm3 g−1.
The magnetic property of Fe3O4/GO and Fe3O4/GO/PW were measured using VSM (Fig. 7). The magnetic measurement (Ms) of Fe3O4/GO and Fe3O4/GO/PW are about 60 and 30 emu g−1, respectively. There is no hysteresis, proposing that Fe3O4/GO/PW is superparamagnetic.
In the following, the catalytic activities, leaching and reusability of three different magnetic catalysts were compared using the reaction of benzaldehyde and dimedone as a model system in water (Scheme 2).
Results show that higher catalytic activity in short reaction time was found in the presence of the Fe3O4/GO/PW (Fig. 8).
The reusability and leaching of three different magnetic catalysts were studied by employing recovered catalysts in the model reaction (Fig. 9a and b). For Fe3O4/GO/PW, the interacting species were well dispersed on the support surface and leaching was low during the reaction which confirmed by ICP-AES measurement as well. So, the Fe3O4/GO/PW catalyst could be recovered and reused several times; only 4.9% of the initial PW content totally leached into the reaction mixture during four successive runs. The yield of the product was not substantially changed (10% difference) after the fourth run. Fe3O4/GA/PW and PW/SMNs could be reused at least four times, albeit with slightly decrease in activities compared to Fe3O4/GO/PW. This behavior may indicate that some leaching of the active species should occur during the subsequent reaction cycles which confirmed by the ICP-AES measurement. As a result, it seems that 11.6% and 26.2% of the PW were leached out from the Fe3O4/GA/PW and PW/SMNs surface during four runs (Fig. 9b).
Another test was performed to check if this active catalyst, Fe3O4/GO/PW, is indeed heterogeneous (Fig. 10). After the hot filtration no further conversion was observed in the model reaction as a proof that no homogeneous catalyst was present in the reaction mixture.
According to the results obtained above, future experiments were carried out using Fe3O4/GO/PW as the best catalyst. The effect of the catalyst loading and reaction temperature on the efficiency was evaluated in the model reaction (Table 2). It was observed that the substrates did not react at room temperature. Whereas by increasing the temperature up to 70 °C, a significant improvement was observed and yield of the product was improved to 95% it may be because of the low solubility of dimedone in the reaction mixture at room temperatures (Table 2, entries 1 and 2). The effect of the catalyst loading was also studied. Decreasing in the catalyst quantity from 0.04 to 0.02 g had no effect on product yield or reaction time. Further decrease from 0.02 to 0.01 g in the catalyst quantity led to increasing in time of the reaction (Table 2, entries 2–4). It should be noted that the yield of product was 22% after 10 min when the reaction was performed in the presence of Fe3O4/GO only. In addition, the reaction did not proceed in the absence of the catalyst.
| Entry | Catalyst | Temp. (°C) | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | Fe3O4/GO/PW (0.04 g) | 25 | 30 | — |
| 2 | Fe3O4/GO/PW (0.04 g) | 70 | 10 | 95 |
| 3 | Fe3O4/GO/PW (0.02 g) | 70 | 10 | 95 |
| 4 | Fe3O4/GO/PW (0.01 g) | 70 | 10 | 60 |
| 5 | Fe3O4/GO (0.02 g) | 70 | 10 | 22 |
| 6 | — | 70 | 30 | — |
Encouraged by these results; we used the Fe3O4/GO/PW catalyst in the reaction of dimedone with various aromatic aldehydes to synthesize structurally diverse DOX; the results are summarized in Table 3.
| Entry | R | Producta | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a All products were identified by comparing their spectral data with those of the authentic samples.62b Isolated yields.c Refer to large scale syntheses. | ||||
| 1 | H |  |
10 | 95 |
| 2 | H |  |
30 | 90 |
| 3c | 4-Cl |  |
15 | 96 |
| 4 | 4-Br |  |
15 | 96 |
| 5 | 4-OH |  |
20 | 84 |
| 6 | 4-NO2 |  |
10 | 98 |
| 7 | 3-NO2 |  |
15 | 92 |
| 8 | 2-NO2 |  |
20 | 93 |
| 9 | 4-OCH3 |  |
15 | 90 |
| 10 | 3,4-OCH3 | 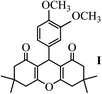 |
15 | 85 |
| 11 | 2,5-OCH3 |  |
20 | 84 |
| 12 | 4-NMe2C6H4 |  |
12 | 95 |
According to the literature63 and results obtained in our experiments, a mechanism is proposed for the synthesis of DOXs as shown in Scheme 3. Initially, the acid catalyst was protonated the carbonyl group of the aldehyde and converted the aldehyde into a convenient electrophile. Then intermediate I was formed via condensation of dimedone with the aromatic aldehyde. Subsequently, intermediate II was produced through combination of a second activated dimedone with intermediate I. Finally, removal of one water molecule of intermediate II led to cyclization and followed by final formation of xanthene products.
Conclusions
Two novel magnetic nanocomposite, Fe3O4/GO/PW and Fe3O4/GA/PW was successfully synthesized through one-step chemical coprecipitation and coprecipitation-solvothermal methods, respectively. Evaluation of these two new synthesized catalysts in term of synthetic methods, activities, leaching and recyclability, showed that Fe3O4/GO/PW has better property than Fe3O4/GA/PW for more investigations. Fe3O4/GO/PW was characterized by FTIR, XRD, VSM, TEM, SEM, EDX, BET and laser particle size analyzer. Fe3O4/GO/PW could be separated rapidly under external magnet; this is an important advantage of the use of a magnetically separable catalyst. The Fe3O4/GO/PW catalyst was used in aqueous synthesis of DOX which are biologically interesting compounds. The method is easily operated, cost-effective, environmentally friendly with high-purity products in excellent yields. The detecting results could indicated our protocol make the reaction suitable for scale-up and commercialization.Acknowledgements
The authors thank the Razi University Research Council for support of this work.Notes and references
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- C. M. Niemeyer, Angew. Chem., Int. Ed., 2001, 40, 4128 CrossRef CAS.
- C. Altavilla, M. Sarno and P. Ciambelli, Chem. Mater., 2009, 21, 4851 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed.
- R. S. Gaster, D. A. Hall, C. H. Nielsen, S. J. Osterfeld, H. Yu, K. E. Mach, R. J. Wilson, B. Murmann, J. C. Liao, S. S. Gambhir and S. X. Wang, Nat. Med., 2009, 15, 1327 CrossRef CAS PubMed.
- D. K. Kim, Y. Zhang, W. Voit, K. V. Rao and M. Muhammed, J. Magn. Magn. Mater., 2001, 225, 30 CrossRef CAS.
- M. S. Nikolic, M. Krack, V. Aleksandrovic, A. Kornowski, S. Förster and H. Weller, Angew. Chem., Int. Ed., 2006, 45, 657719 CrossRef PubMed.
- D. K. Yi, S. T. Selvan, S. S. Lee, G. C. Papaefthymiou, D. Kundaliya and J. Y. Ying, J. Am. Chem. Soc., 2005, 127, 4990 CrossRef CAS PubMed.
- S. Santra, R. Tapec, N. Theodoropoulou, J. Dobson, A. Hebard and W. Tan, Langmuir, 2001, 17, 2900 CrossRef CAS.
- J. Rivas, R. D. SXnchez, A. Fondado, C. Izco, A. J. Garcia-Bastida, J. Garcia-Otero, J. Mira, D. Baldomir, A. GonzXlez, I. Lado, M. A. Lwpez-Quintela and S. B. Oseroff, J. Appl. Phys., 1994, 76, 6564 CrossRef CAS.
- Y. S. Shon, G. B. Dawson, M. Porter and R. W. Murray, Langmuir, 2002, 18, 3880 CrossRef CAS.
- J. H. J. Scott and S. A. Majetich, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 12564 CrossRef CAS.
- K. H. Ang, I. Alexandrou, N. D. Mathur, G. A. J. Amaratunga and S. Haq, Nanotechnology, 2004, 15, 520 CrossRef CAS.
- W. Teunissen, F. M. F. de Groot, J. Geus, O. Stephan, M. Tence and C. Colliex, J. Catal., 2001, 204, 169 CrossRef CAS.
- T. Hayashi, S. Hirono, M. Tomita and S. Umemura, Nature, 1996, 381, 772 CrossRef.
- R. Nesper, A. Ivantchenko and F. Krumeich, Adv. Funct. Mater., 2006, 16, 296 CrossRef CAS.
- S. I. Nikitenko, Y. Koltypin, O. Palchik, I. Felner, X. N. Xu and A. Gedanken, Angew. Chem., 2001, 113, 4579 CrossRef.
- J. Geng, D. A. Jefferson and B. F. G. Johnson, Chem. Commun., 2004, 23, 2442 RSC.
- M. Bystrzejewski and M. H. Rümmeli, Pol. J. Chem., 2007, 81, 1219 CAS.
- W. S. Seo, J. H. Lee, X. Sun, Y. Suzuki, D. Mann, Z. Liu, M. Terashima, P. C. Yang, M. V. Mcconnell, D. G. Nishimura and H. Dai, Nat. Mater., 2006, 5, 971 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324 CrossRef CAS PubMed.
- Y. Huang, J. Liang and Y. Chen, Small, 2012, 25, 1805 CrossRef PubMed.
- A. Ghosh and Y. H. Lee, ChemSusChem, 2012, 12, 480 CrossRef PubMed.
- X. C. Gui, A. Y. Cao, J. Q. Wei, H. B. Li, Y. Jia, Z. Li, L. L. Fan, K. L. Wang, H. W. Zhu and D. H. Wu, ACS Nano, 2010, 4, 2320 CrossRef CAS PubMed.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301 CrossRef CAS PubMed.
- B. Das, B. Choudhury, A. Gomathi, A. K. Manna, S. K. Pati and C. N. R. Rao, ChemPhysChem, 2011, 12, 937 CrossRef CAS PubMed.
- Y. Zhao, C. G. Hu, Y. Hu, H. H. Cheng, G. Q. Shi and L. T. A. Qu, Angew. Chem., Int. Ed., 2012, 51, 11533 CrossRef.
- Z. Chen, C. Xu, C. Ma, W. Ren and H. Cheng, Adv. Mater., 2013, 25, 1296 CrossRef CAS PubMed.
- J. Li, J. Li, H. Meng, S. Xie, B. Zhang, L. Li, H. Ma, J. Zhang and M. Yu, J. Mater. Chem. A, 2014, 2, 2934 CAS.
- D. D. Nguyen, N. H. Tai, S. B. Lee and W. S. Kuo, Energy Environ. Sci., 2012, 5, 7908 CAS.
- T. A. Schaedler, A. J. Jacobsen, A. Torrents, A. E. Sorensen, J. Lian, J. R. Greer, L. Valdevit and W. B. Carter, Science, 2011, 334, 962 CrossRef CAS PubMed.
- K. H. Kim, Y. Oh and M. F. Islam, Nat. Nanotechnol., 2012, 7, 562 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimmey, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Natural, 2006, 442, 282 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- G. D. Yadav and G. George, J. Mol. Catal. A: Chem., 2008, 292, 54 CrossRef CAS.
- G. D. Yadav, S. S. Salgaonkar and N. S. Asthana, Appl. Catal., A, 2004, 265, 153 CrossRef CAS.
- H. Eshghi, A. Javid, A. Khojastehnezhad, F. Moeinpour, F. F. Bamoharram, M. Bakavoli and M. Mirzaei, Chin. J. Catal., 2015, 36, 299 CrossRef CAS.
- E. Rafiee, M. Khodayari, S. Shahebrahimi and M. Joshaghani, J. Mol. Catal. A: Chem., 2011, 351, 204 CrossRef CAS.
- Y. C. Kim, J. Y. Jeong, J. Y. Hwang, S. D. Kim, S. C. Yi and W. J. Kim, J. Membr. Sci., 2008, 325, 252 CrossRef CAS.
- I. V. Kozhevnikov, Catalysis by Polyoxometalates, 2, Wiley, Chichester, 2002 Search PubMed.
- I. V. Kozhevinkov, Chem. Rev., 1998, 98, 171 CrossRef.
- E. Rafiee, F. Shahbazi, M. Joshaghani and F. Tork, J. Mol. Catal. A: Chem., 2005, 242, 129 CrossRef CAS.
- E. Rafiee and S. Eavani, Green Chem., 2011, 13, 2116 RSC.
- Z. Hernandez-Gallegos, P. A. Lehman-F, E. Hong, F. Posadas and E. Hernandez-Gallegos, Eur. J. Med. Chem., 1995, 30, 355 CrossRef CAS.
- G. I. Shakibaei, P. Mirzaei and A. Bazgir, Appl. Catal., A, 2007, 325, 188 CrossRef CAS.
- A. K. Bhattacharya, K. C. Rana, M. Mujahid, I. Sehar and A. K. Saxena, Bioorg. Med. Chem. Lett., 2009, 19, 5590 CrossRef CAS PubMed.
- M. A. Naik, D. Sachdev and A. Dubey, Catal. Commun., 2010, 11, 1148 CrossRef CAS.
- K. Gong, D. Fang, H. L. Wang, X. L. Zhou and Z. L. Liu, Dyes Pigm., 2009, 80, 30 CrossRef CAS.
- W. Shen, L. M. Wang, H. Tian, J. Tang and J. J. Yu, J. Fluorine Chem., 2009, 130, 522 CrossRef CAS.
- M. Hong and C. Cai, J. Fluorine Chem., 2009, 130, 989 CrossRef CAS.
- S. K. Ko and C. F. Yao, Tetrahedron Lett., 2006, 47, 8827 CrossRef CAS.
- M. Kidwai and D. Bhatnagar, Tetrahedron Lett., 2010, 51, 2700 CrossRef CAS.
- W. S. Hummer Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- J. Li, S. Zhang, C. Chen, G. Zhao, X. Yang, J. Li and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4991 CAS.
- Y. S. Kang, S. Risbud, J. F. Rabolt and P. Stroeve, Chem. Mater., 1996, 8, 2209 CrossRef CAS.
- E. Rafiee and M. Khodayari, J. Mol. Catal. A: Chem., 2015, 398, 336 CrossRef CAS.
- H. A. Becerril, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, ACS Nano, 2008, 2, 463 CrossRef CAS PubMed.
- M. T. Pope, in Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin, 1983, p. 23 Search PubMed.
- E. Kazemi, S. Dadfarnia and A. M. HajiShabani, Talanta, 2015, 141, 273 CrossRef CAS PubMed.
- E. Rafiee, S. Eavani, S. Rashidzadeh and M. Joshaghani, Inorg. Chim. Acta, 2009, 362, 3555 CrossRef CAS.
- S. Kantevari, R. Bantu and L. Nagarapu, J. Mol. Catal. A: Chem., 2007, 269, 53 CrossRef CAS.
- B. R. Madje, M. B. Ubale, J. V. Bharad, M. S. Shingare and S. Afr, J. Chem., 2010, 63, 36 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |