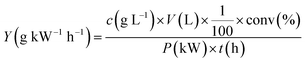DOI:
10.1039/C6RA02807A
(Paper)
RSC Adv., 2016,
6, 28994-29002
Catalytic degradation of 4-chlorophenol with La/TiO2 in a dielectric barrier discharge system†
Received
30th January 2016
, Accepted 15th March 2016
First published on 16th March 2016
Abstract
Lanthanum (La) doped titanium dioxide (TiO2) introduced to the dielectric barrier discharge (DBD) system was successfully used to degrade 4-chlorophenol (4-CP). The photocatalytic materials were characterized by XRD, SEM, EDX and DRS techniques. The influence of the La doping ratio and pH on the degradation of 4-CP in the combined system of photocatalysis and plasma were investigated to evaluate the feasibility of the mixed degradation system. The 10 wt% La/TiO2 showed the highest percentage of 4-CP degradation (99.0%) and maximum rate constant (11.89 × 10−3 s−1). It was also found that the catalytic activity of 10La/TiO2 was higher than pure synthesized TiO2. Doped La effectively reduces the band gap, amends the surface and optimizes the crystal form of TiO2. Higher degradation efficiency of 4-CP was observed at higher pH values. The efficiency was 99.9% at pH 10.0 in this treatment system, while a decrease was observed at pH 2.0. Catechol, hydroquinone, benzoquinone and carboxylic acid were identified as the predominant aromatic intermediates for the degradation of 4-CP, and finally, transformed into CO2 and H2O.
1. Introduction
As we known, 4-chlorophenol (4-CP) is an important environmental pollutant due to its toxicity and persistency, and it is frequently found in the effluents of petrochemical, pesticide, plastic, kraft mill and other organic chemical production industries and research centers.1–3 Conventional treatment methods such as biological treatment, chlorination and adsorption cannot completely remove contaminants or they generate carcinogenic by-products.4 Thus, as effective methods for treating a wide range of pollutants, photocatalysis and plasma have received extensive attention.5–7
Photocatalytic degradation of toxic organic compounds is very promising for the purification and treatment of industrial wastewater.8 As early as 1976, Carey et al. were the first to report photocatalytic degradation of organic pollutants.9 Now, the principles and applications of photocatalysis have been excellently reviewed, especially its utilization for environmental treatment and the synthesis of fine chemicals. Titanium dioxide (TiO2) has by far most often been used and investigated as a photocatalyst due to its unique properties, such as high refractive index, excellent optical transmittance in the visible and near-infrared regions, high photo-chemical and corrosive resistance, high dielectric constant and photocatalytic activity.10–12 However, the drawback that limits its practical application is TiO2 has a large band gap (about 3.2 eV). The band edge absorption threshold prevents TiO2 from using visible light, so the main excitation wavelength is less than 400 nm.6,10,13–15 In order to circumvent the limitation, doping of metals or nonmetals has been attended to modify the photoactivity of TiO2 via red shifting its absorption wavelength to visible light region.6,13,15 Some studies have reported that lanthanon (La) can effectively improve the photocatalytic activity of TiO2 due to the transitions of 4f electrons, which can improve the separation efficiency of photo-induced electron–hole pairs of TiO2 to enhance the photoreactivity.13,16
Dielectric barrier discharge (DBD) plasma as one of advanced oxidation processes (AOPs), has received widespread attention in the field of wastewater treatment due to effective degradation capacity. During electrical discharges process, many chemically active species can be generated such as high-energy electrons, radicals (e.g. HO˙, HO2˙, O˙, etc.), ozone, neutral molecules (excited state) and ions, and ultraviolet light also can be produced, these are beneficial to optimize the degradation process. The detailed formation mechanisms for these species during the discharge are as follows (eqn 1–10):17–21
| |
 | (1) |
| | |
O2− + H2O → HO˙2 + OH−
| (5) |
| |
 | (6) |
| | |
O3 + H2O2 → HO˙ + O2 + HO˙2
| (7) |
| | |
O3 + HO˙2 → HO˙ + O2 + O2−
| (8) |
| |
 | (9) |
| |
 | (10) |
However, in practical application, single DBD plasma processing system has several weaknesses like low energy yields and low mineralization rates.5,12,22–24
The objective of this study is to explore the effect of lanthanum doped titanium oxide on the catalytic degradation in DBD plasma system. In order to clarify the role of lanthanum in combined degradation process, we experimented with different lanthanum doping on the removal of 4-CP, and investigated characteristics of La/TiO2 catalysts. Thereby, we confirmed the optimum doping amount of lanthanum and demonstrated La/TiO2 catalytic advantages. In addition, the effect of pH value was evaluated to elucidate the enhanced effect of coupled system on the degradation 4-CP. Moreover, the possible reaction pathways of 4-CP degradation were also presented.
2. Material and method
2.1 Materials
The regents used in this study were analytical grade: tetrabutyl titanate (Tianjin Kermel Chemical Industry Co., Ltd.), lanthanum nitrate (Tianjin Fuchen Chemical Industry Co., Ltd.), 4-chlorophenol (Chengdu Xiya Chemical Industry Co., Ltd.) absolute ethanol and anhydrous acetic acid (Tianjin Fuyu Chemical Industry Co., Ltd.), honeycomb ceramic (Al2O3 content > 95%, porous size 1 mm, height 10 mm, diameter about 100 mm, purchased from shanghai industrial ceramics Co., Ltd.), NaOH and HCl (adjust the pH value of solution), Na2HPO4 (adjust the conductivity of solution).
2.2 Catalyst preparation
The steps for the preparation of the catalyst are as follows:13
(1) The catalyst was a lanthanum doped titanium oxide with different mass percentage of lanthanum (0%, 2%, 5%, 10% and 15%) prepared by the sol–gel method. Briefly 10 mL of tetrabutyl titanate was dissolved in 50 mL of absolute ethanol (solution A).
(2) In parallel, corresponding weight of La(NO3)·6H2O was dissolved in 10 mL distilled water and then added to 40 mL of ethanol and 10 mL of anhydrous acetic acid (solution B). Solution A was then added dropwise to a beaker containing solution B.
(3) Put solution A on a magnetic stirrer and add solution B into solution A, keep a dropping speed of 1 drop/10 s (to inhibit the hydrolysis rate of tetrabutyl titanate). After dropping, continue stirring for 3–5 min to get the transparent sol.
(4) Put a dry and clean honeycomb ceramic into the sol for 5 min, then keep the pulling speed of 2–4 mm s−1, stove it for 10 min in the drying oven at 80 °C, repeat the procedure for four times.
(5) Calcine step-by-step in the muffle: heat up from room temperature to 110 °C (0.25 °C min−1), from 110 to 210 °C (0.25–0.5 °C min−1), from 210 to 500 °C (0.5–1 °C min−1), keep 500 °C for 3–4 h to form catalyst.
2.3 Experimental set up
The device was used to conduct the experiments, which is shown like the schematic diagram (Fig. 1). The high-voltage power supply (CTP-2000 K) used in present study is the same as that in our previous works,25 the output power was set to 100 W. The structure of reactor is similar to flow sedimentation tank (height 700 mm, inner diameter 100 mm and outer diameter 140 mm), and the buffering of baffle plate and honeycomb ceramic (height 10 mm, diameter about 100 mm, pore diameter 1 mm) can ensure smooth surface. The two parts of electrode are a discharge electrode and a ground electrode. A piece of quartz glass (diameter 90 mm) is used as isolation medium and is located below the discharge electrode. The peristaltic pump can keep 4-chlorophenol solution (100 mg L−1) circulating. The whole experiment process was conducted at normal pressure and temperature.
 |
| | Fig. 1 Schematic diagram of the experimental apparatus. | |
2.4 Analysis methods
The concentration of 4-chlorophenol and products were analyzed by high efficiency liquid chromatography (HPLC, Thermo Ultimate 3000) equipped with C-18 column (Atlantis). The mobile phase used in HPLC was a mixed solvent of methanol and water (80/20, v/v) with a flow rate of 0.2 mL min−1. The dissolved organic carbon (DOC) determined as total content of organic carbon (TOC) was measured with a Shimadzu TOC-VCPH analyzer. Powder X-ray diffractions of samples were obtained using a Rigaku D/MAX-rA diffractometer with the Cu Kα (λ = 0.154184 nm) radiation. Samples were scanned from 20 to 80 at a rate of 8° min−1. The diffractometer was performed at 40 kV and 40 mA. The surface structures of ceramics attached with catalyst were observed with scanning electron microscope (SEM, JSM-6700F, Shimadzu). The optical absorption property was measured by using ultraviolet visible diffuse reflectance spectroscopy (UV-vis DRS, UV-2550, Shimadzu) at room temperature in the wavelength region between 220 and 800 nm. Ultrafine BaSO4 powder supplied by Shimadzu Company was used as a reference.
The efficiency of 4-CP degraded was determined from the following equation:
| |
 | (11) |
where
C0 is the initial concentration of 4-CP and
Ct is the concentration of 4-CP after ‘
t’ minutes.
The degradation reactions of 4-CP obeyed the apparent first order law. The kinetic equation could be expressed as follow:
| |
 | (12) |
where
C0,
Ct,
kcp and
t are the initial concentration of 4-CP, the concentration of 4-CP after ‘
t’ minutes, the rate constant and reaction time, respectively.
The yield26 can also well reflect the degradation of pollutant, which defined as the amount of 4-CP decomposed per unit of energy consumed in the process:
| |
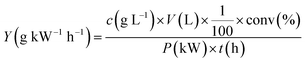 | (13) |
where
c is the initial concentration of 4-CP in solution,
V is the solution volume, conv is the degradation efficiency,
P is the average power in the discharge and
t is the degradation time.
3. Result and discussion
3.1 Effect of La doping for 4-chlorophenol degradation
In the experimental initial condition, the initial 4-CP concentration, solution volume, pH value were 100 mg L−1, 1000 mL, 6.0, respectively. As shown in Fig. 3a, the degradation efficiency values of 4-CP in DBD plasma system with catalyst were higher than that without catalyst.
 |
| | Fig. 2 Photographs of plasma discharges for system equipped with porous ceramic segment. | |
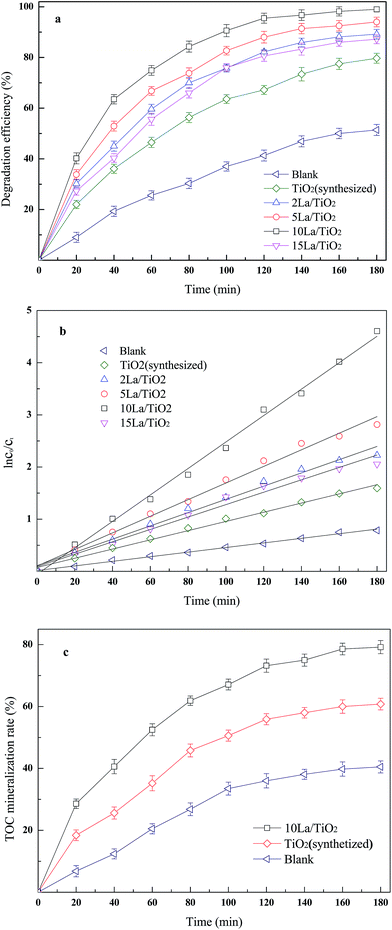 |
| | Fig. 3 Effect of various La doping 4-CP degradation (a) and corresponding kinetic constants (b) TOC mineralization rates of optimum La doping, synthetized TiO2 and blank (c). | |
In fact, the reactive species (HO˙, O˙, O3, etc.) generated during discharge process of plasma can directly oxidize organic pollutants. However, the power imported in a single plasma system is wasted, leading to low energy yield. For example, Krause et al. used single corona system to treat clofibric acid and phenol and energy yields were 17 × 10−3 g kW−1 h−1 and 15 × 10−3 g kW−1 h−1, respectively.27 By contrast, the DBD system with introducing catalysts could effectively reduce energy waste. Table 1 summarizes rate constants, degradation efficiency values and energy yields of the degradation of 4-CP under different catalyst conditions. It can easily be found that the DBD plasma process in combination with TiO2 exhibited higher values of energy yields (27 g kW−1 h−1) than one without catalyst (17 g kW−1 h−1) of 4-CP degradation, and higher degradation efficiency and rate constant also revealed the advantages of combination of plasma and catalytic.
Table 1 Kinetic constants, degradation efficiency values and energy yields of 4-CP with different catalysts
| Catalysts |
kcp (10−3 s−1) |
R2 |
Degradation efficiency (%) |
Y (g kW−1 h−1) |
| Blank |
4.41 |
0.995 |
51.4 |
17 |
| TiO2 (synthesized) |
8.81 |
0.993 |
79.7 |
27 |
| 2La/TiO2 |
12.70 |
0.987 |
89.2 |
30 |
| 5La/TiO2 |
15.92 |
0.990 |
94.0 |
31 |
| 10La/TiO2 |
25.35 |
0.996 |
99.0 |
33 |
| 15La/TiO2 |
11.89 |
0.981 |
87.2 |
29 |
Fig. 3a also shows the effect of La dopant on degradation of 4-CP. The weight ratio of La doping in the sample was from 0% to 15%. It is obviously that introducing La into TiO2 always enhances the degradation of 4-CP. The degradation efficiency and energy yield of 4-CP augmented with increasing La ratio, reaching the maximum value of 99.0% and 33 g kW−1 h−1 with sample doping 10 wt% La which was better than Tezuka et al. reported.28 Further increase of La proportion led to a decrease of degradation efficiency from 99.0% to 87.2%, but still higher than 79.7% of synthesized TiO2. Meanwhile, the trend of degradation kinetic (Fig. 3b) can also demonstrate the same conclusion that the presence of the dopant La resulted higher rate constant kcp compared with that without La. The constants varied from 8.81 × 10−3 s−1 (R2 = 0.993) to 25.35 × 10−3 s−1 (R2 = 0.996) for 0 wt% La and 10 wt% La, respectively, and the constant of 15 wt% La sample is 11.89 × 10−3 s−1 (R2 = 0.981). As a result of this, mixed treatment was much more effective than previous plasma studies about 4-CP. Experimental results above could indicate that La plays a positive role in degradation process, and confirmed that excessive dopant La inhibits the degradation process. Actually, the decrease in degradation efficiency could probably be explained by partial blocking of the active species of TiO2 due to the formation of larger La2O3 particles at higher La2O3 loading. In addition, tendency of La2O3 to form larger particles on TiO2 surface has also caused lower the conversion of 4-CP.7 The decrease of degradation with higher La proportion in the composite sample is also considered to be related to increased absorbing and scattering of photon by surplus lanthanum.29,30
The total organic carbon (TOC) was monitored at different time intervals to examine the mineralization of organic matter, among no-catalyst, synthesized TiO2 and 10 wt% La/TiO2. Although the concentration of 4-CP could be almost completely decomposed in the presence of 10 wt% La dopant, the mineralization of 4-CP was not complete (79.2%). As depicted in Fig. 3c, the mineralization efficiency of single plasma was the lowest one. And TiO2 was lower than that of 10 wt% La doped TiO2, and achieving the highest mineralization of TiO2 just required 80 min with 10 wt% La doped TiO2. Also, the mineralization behavior followed apparent first-order rate equation. The constants were 8.71 × 10−3 s−1, 5.33 × 10−3 s−1 and 3.15 × 10−3 s−1 of 10 wt% La/TiO2, synthesized TiO2 and no-catalyst, respectively.
Therefore, the experimental results could prove that the optimum La doping weight proportion was 10%, and synthesized TiO2 was found to be less active than 10 wt% La doped TiO2.
3.2 Reproducibility of La/TiO2 catalyst
A four-cycle experiment had revealed the reproducibility of the catalyst for 4-CP degradation. Each cycle was performed under identical conditions of 100 mg L−1 4-CP, 10 wt% La/TiO2, pH = 6.0, and 180 min runtime. After the four cycles, the corresponding efficiency values are observed in Fig. 4. The efficiency values were 99.0%, 96.9%, 94.1%, and 92.3% for the first to the fourth runs, respectively. A gradually decreasing trend can be found from the results of degradation efficiency, but the differences among the four were not obvious. Therefore, the catalyst with 10 wt% La doped TiO2 could be accepted as the repeatability and stability were great for catalytic degradation experiment.
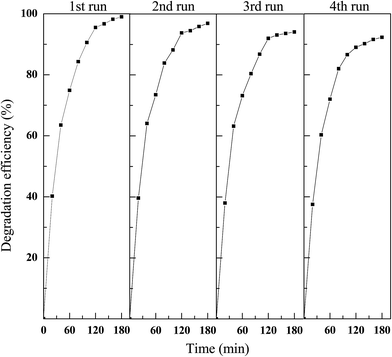 |
| | Fig. 4 The reproducibility of the La-doped TiO2 for degradation of 4-chlorophenol. | |
3.3 X-ray diffraction (XRD)
The XRD diffraction patterns of synthesized TiO2 and different La doping TiO2 catalysts were obtained. As shown in Fig. 5, the obvious diffraction peaks of samples illustrate that the crystal structure was thoroughly organized. The anatase phase structure of TiO2 could be detected in both synthesized TiO2 and composite catalysts such as the planes (101), (004), (200), (211) and (204) at 2θ values of ca. 25.3°, 38.0°, 48.0°, 54.1° and 62.6° respectively.31 After lanthanum doping, the main diffraction peak (101) at 25.3° of anatase phase TiO2 was not shifted to other angles, but the intensities of peaks were lower than those found in synthesized TiO2. This could be attributed to the presence of foreign ions in the catalyst.32 Among all doped samples, the peak of anatase in 10 wt% La/TiO2 was the most regular and the most similar to synthesized TiO2. The conspicuous diffraction peaks of rutile phase almost disappeared from diffraction patterns of La doping TiO2, namely, planes (110) and (211) at 2θ values of ca. 27.4° and 54.3°. Thus, the doping of La was shown to inhibit the anatase-rutile polymorphic transformation and expected to play a significant control in the selective crystallisation of anatase phase during sol–gel process.6,33 The ionic radius of La3+ is 0.115 nm and seems to be larger than pore diameter of the titania to be introduced in lattice of TiO2. It is difficult to occur that La3+ replaces Ti4+ from TiO2 lattice. Thus, the diffraction peaks of lanthanum modified TiO2 were nearly same as that of synthesized TiO2 which indicates that La3+ is more likely to be found as dispersed metal oxides within the crystal matrix or they are dispersed on the surface of TiO2.11,16,30,34
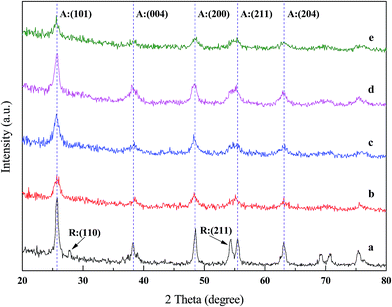 |
| | Fig. 5 XRD patterns of (a) synthetized TiO2 (b) 2La/TiO2 (c) 5La/TiO2 (d) 10La/TiO2 (e) 15La/TiO2. | |
3.4 Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX)
Both synthesized TiO2 and La/TiO2 were adhered to the honeycomb ceramics during sol–gel process. After calcination, with catalysts deposited on the ceramics, the surface of ceramics were characterized using scanning electron microscopy (SEM) combined with energy dispersive X-ray (EDX) analysis. As shown in Fig. 6, a large number of cracks were formed on the surface of synthesized TiO2, and adhesive layer of TiO2 was divided into many small areas (Fig. 6a). On the contrary, TiO2 with doped La was more uniformly distributed on the surface of ceramics. With the increase of the amount of La, the surface of catalysts became more and more smooth. This phenomenon might be attributed to the modification of La on TiO2. And 10 wt% La doping achieved optimal status (Fig. 6d). So the specific surface area of impure catalyst was increased, and the catalytic performance was improved. However, excessive La doping could cause adverse effect, leading to cracks on the surface of catalyst (Fig. 6e).
 |
| | Fig. 6 SEM images of synthetized TiO2 (a), 2 wt% La/TiO2 (b), 5 wt% La/TiO2 (c), 10 wt% La/TiO2 (d), 15 wt% La/TiO2 (e) and EDX patterns of 10 wt% La doped TiO2 (f). | |
The energy dispersive X-ray (EDX) microanalysis was carried out to prove the presence of La in TiO2 (Fig. 6f). The weight percentages of Ti and La in 10 wt% La/TiO2 resulted equal to 64.45 and 7.33, respectively. It reveals that the actual result was basically consistent with the theoretical doping ratio (10 wt%). And the intense peak was assigned to TiO2 in the bulk form and the less intense peak is assigned to surface TiO2.4
3.5 UV-vis spectra and band gap energy
The UV-vis diffuse reflectance spectra of synthetized TiO2 and La/TiO2 with different La doping are shown in Fig. 7. By analyzing the spectra, we can ascertain the responsivity of each sample of La modified and synthetized TiO2 to the visible light, and calculate the band gap energy (Table 2). The band gap energy is determined from Planck's relation:where h is Planck constant (6.62 × 10−34 J s), EG is band gap energy (eV, 1 eV = 1.602 × 10−19 J), c is speed of light (3 × 10−8 m s−1) and λ is wavelength (nm).
 |
| | Fig. 7 UV-vis diffuse reflectance spectra of La modified TiO2. | |
Table 2 Wavelength and energy of the band gap of TiO2 doped samples prepared
| Solid |
Wavelength (nm) |
EG (eV) |
| TiO2 (synthesized) |
435 |
2.85 |
| 2La/TiO2 |
483 |
2.57 |
| 5La/TiO2 |
524 |
2.37 |
| 10La/TiO2 |
572 |
2.17 |
| 15La/TiO2 |
561 |
2.21 |
These are gathered in Table 2. We can observe a great correlation between the light properties and the doping concentration of La. The synthesized TiO2 had the absorption of about 435 nm (EG ∼ 2.85 eV). By contrast, the samples of La doped TiO2 showed extensive absorption spectra in visible range of 400–600 nm. It is also confirmed that the longest wavelength (λ ∼ 572 nm) and minimum band gap energy (EG ∼ 2.17 eV) appeared in the sample of 10 wt% La doped TiO2.
The red shift phenomenon in Fig. 7 could be ascribed to formation of impurity levels within the band gap of TiO2 due to La doping. The dopant might induce the generation of oxygen vacancies during synthesis. As a consequence, the electronic transition between these impurity levels and valence or conduction band effectively enhances the light absorption in visible light region.11,16 The surface TiO2 might be covered by redundant La, the synergism of TiO2 and La is inhibited and hence excessive dopant can not broaden absorption region and reduce the band gap energy. This suggests, that the absorption property of visible light can impact the activity of 4-CP degradation.10
3.6 Effect of pH for 4-chlorophenol degradation
It was reported that the initial pH value is a significant processing factor affecting the removal efficiency of contaminants in the DBD system. Wastewater is often discharged with various pH values from industrial process. The pH values were adjusted with 0.1 N NaOH or 0.1 N HCl to reach the desired pH values. The degradation efficiencies of 4-CP at several initial pH values are shown in Fig. 8a. Nearly complete degradation of 4-CP was observed within 100 min at pH = 10.0. It could be clearly identified that decreasing the pH values led to prolonged degradation time, even depressed degradation efficiency. 83.3% removal of the original 4-CP within 180 min was observed when pH value decreased to 2.0. Few researchers have reported that the surface of TiO2 carries positive charge (pHzpc = 6.25) under low pH condition, whereas the molecules of chlorophenols and intermediates are mainly negative and neutral. Therefore, low pH can promote the adsorption of pollutants on surface of TiO2, thereby enhancing the degradation efficiency.35–37 However, previous studies also showed that the majority of adsorption sites of TiO2 are occupied by water molecules in aqueous, very few chlorophenols can be adsorbed by TiO2.38,39 So 4-CP is more easily degraded under alkaline condition,5,39–42 which is consistent with our results. In dielectric barrier discharge system, electric current breakdown of air layer can generate ozone and ultraviolet light. As Fig. 2 shows ultraviolet can be found in our dielectric barrier discharge system. Then hydroxyl radicals (HO˙) and hydrated electrons (e−aq) can be formed when water is ionized by electric current and irradiated with high energy ultraviolet light. Meanwhile, direct electron transfer and adsorption reactions are induced on the surface of TiO2 under ultraviolet light and visible light.39,41 Consequently, higher pH value can provide higher concentration of hydroxyl ions to react with holes to form hydroxyl radicals, subsequently enhancing the photodegradation rate of 4-CP:43| | |
TiO2 + hν → TiO2(h+ + e−)
| (15) |
| | |
OH− + hν → HO˙ + e−aq
| (17) |
 |
| | Fig. 8 The degradation of 4-chlorophenol in combined system under different initial pH values (a) and changes of pH during 4-chlorophenol degradation process (b). | |
The disappearance of 4-CP could be attributed to synergistic reaction of the above the factors.
The changes of pH values are shown in Fig. 8b. As can be seen, the solution pH value was decreased during 4-CP degradation process, which indicated that the degradation of 4-CP was an acidification process. That might be the reason why the degradation efficiency increased slowly in the late phase of degradation reaction. Under the DBD condition, Cl− could be generated in 4-CP dechlorination process, in the meantime, NO3− could be produced by N2.44 The formation of HCl and HNO3 caused the decreasing pH of solution.
3.7 Reaction pathways for 4-chlorophenol degradation
According to the analysis of degradation intermediates and also taking degradation mechanism suggested by former workers into consideration,1,5,24,28,45 we proposed a mechanism for the oxidation of 4-CP (Fig. 9). Due to electrophilic of hydroxyl radical and electron-donating character of OH group, the para- and ortho-position are more vulnerable to the attack of hydroxyl radicals, forming catechol and hydroquinone with loss of chlorine. With further oxidation, catechol and hydroquinone are oxidized into two types of benzoquinone. Then the rings are broken to generate the corresponding carboxylic acid. Gradually, the carboxylic acids are mineralized to carbon dioxide and water. Under acidic condition, meta-position also could be attacked to produce malic acid, and eventually is completely mineralized as water and carbon dioxide.
 |
| | Fig. 9 Proposed reaction pathway for degradation of 4-chlorophenol. | |
4. Conclusions
The results obtained in this study demonstrate that La doping ratio and pH have great effects on degradation of 4-CP in combined system of photocatalysis and plasma. Doped La can effectively improve the catalytic activity of pure synthesized TiO2. The 10 wt% La/TiO2 exhibited highest degradation efficiency (99.0%), maximum rate constant (11.89 × 10−3 s−1) and best mineralization level (79.2%). 10 wt% La doping can decrease the band gap from 2.85 eV to 2.17 eV, inhibit the anatase–rutile polymorphic transformation and amend surface structure of TiO2. And the repeatability and stability of La/TiO2 catalytic honeycomb ceramics are great for catalytic degradation experiment. High pH value was conducive to enhance degradation efficiency of 4-CP. The efficiency at pH 10.0 (99.9%) was 1.2 times as much as pH 2.0 (83.3%). In this combined system, the transformation of 4-CP does not only involve hydroxyl radical and ozone oxidation, but also include direct electron transfer. Catechol, hydroquinone, benzoquinone and carboxylic acid were identified as the predominant aromatic intermediates for the degradation of 4-CP, and finally, decomposed CO2 and H2O.
Acknowledgements
The research was supported by Technological Progress Plan of Shandong, grant no. 2011GGE27048, China.
References
- B. Deka and K. G. Bhattacharyya, J. Environ. Manage., 2015, 150, 479–488 CrossRef CAS PubMed.
- N. Kashif and F. Ouyang, J. Environ. Sci., 2013, 25(2), 399–404 CrossRef.
- A. T. Nguyen and R. S. Juang, J. Environ. Manage., 2015, 147, 271–277 CrossRef CAS PubMed.
- N. Venkatachalam, M. Palanichamy, B. Arabindoo and V. Murugesan, J. Mol. Catal. A: Chem., 2007, 266(1), 158–165 CrossRef CAS.
- X. H. Chen, W. J. Bian, X. H. Song, D. Q. Liu and J. Zhang, Sep. Purif. Technol., 2013, 120, 102–109 CrossRef CAS.
- N. Venkatachalam, M. Palanichamy and V. Murugesan, J. Mol. Catal. A: Chem., 2007, 273(1), 177–185 CrossRef CAS.
- G. S. Pozan and A. Kambur, Appl. Catal., B, 2013, 129, 409–415 CrossRef CAS.
- S. J. Tsai and S. Cheng, Catal. Today, 1997, 33(1), 227–237 CrossRef CAS.
- J. H. Carey, J. Lawrence and H. M. Tosine, Bull. Environ. Contam. Toxicol., 1976, 16(6), 697–701 CrossRef CAS PubMed.
- C. Lettmann, K. Hildenbrand, H. Kisch, W. Macyk and W. F. Maier, Appl. Catal., B, 2001, 32(4), 215–227 CrossRef CAS.
- S. N. R. Inturi, T. Boningari, M. Suidan and P. G. Smirniotis, Appl. Catal., B, 2014, 144, 333–342 CrossRef CAS.
- X. L. Hao, M. H. Zhou, Y. Zhang and L. C. Lei, Plasma Chem. Plasma Process., 2006, 26(5), 455–468 CrossRef CAS.
- J. Santiago-Morales, A. Agüera, M. del Mar Gómez, A. R. Fernández-Alba, J. Giménez, S. Esplugas and R. Rosal, Appl. Catal., B, 2013, 129, 13–29 CrossRef CAS.
- B. Palanisamy, C. M. Babu, B. Sundaravel, S. Anandan and V. Murugesan, J. Hazard. Mater., 2013, 252, 233–242 CrossRef PubMed.
- W. Zhang, X. J. Li, G. Jia, Y. F. Gao, H. Wang, Z. Z. Cao, C. H. Li and J. R. Liu, Catal. Commun., 2014, 45, 144–147 CrossRef CAS.
- N. R. Khalid, E. Ahmed, Z. L. Hong and M. Ahmad, Appl. Surf. Sci., 2012, 263, 254–259 CrossRef CAS.
- X. J. Xu, Thin Solid Films, 2001, 390(1), 237–242 CrossRef CAS.
- N. Sano, T. Kawashima, J. Fujikawa, T. Fujimoto, T. Kitai and A. Toyoda, Ind. Eng. Chem. Res., 2002, 41(24), 5906–5911 CrossRef CAS.
- R. Peyrous, P. Pignolet and B. Held, J. Phys. D: Appl. Phys., 1989, 22(11), 1658 CrossRef CAS.
- B. Eliasson, M. Hirth and U. Kogelschatz, J. Phys. D: Appl. Phys., 1987, 20(11), 1421 CrossRef CAS.
- F. Abdelmalek, M. R. Ghezzar, M. Belhadj, A. Addou and J. L. Brisset, Ind. Eng. Chem. Res., 2006, 45(1), 23–29 CrossRef CAS.
- X. L. Hao, M. H. Zhou and L. C. Lei, J. Hazard. Mater., 2007, 141(3), 475–482 CrossRef CAS PubMed.
- D. Zhu, L. Jiang, R. L. Liu, P. Chen, L. Lang, J. W. Feng, S. J. Yuan and D. Y. Zhao, Chemosphere, 2014, 117, 506–514 CrossRef CAS PubMed.
- R. Sauleda and E. Brillas, Appl. Catal., B, 2001, 29(2), 135–145 CrossRef CAS.
- S. P. Li, Y. Y. Jiang, X. H. Cao, Y. W. Dong, M. Dong and J. Xu, Environ. Technol., 2013, 34(12), 1609–1616 CrossRef CAS PubMed.
- M. Magureanu, D. Piroi, N. B. Mandache, V. David, A. Medvedovici and V. I. Parvulescu, Water Res., 2010, 44(11), 3445–3453 CrossRef CAS PubMed.
- H. Krause, B. Schweiger, J. Schuhmacher, S. Scholl and U. Steinfeld, Chemosphere, 2009, 75(2), 163–168 CrossRef CAS PubMed.
- M. Tezuka and M. Iwasaki, Thin Solid Films, 1998, 316(1), 123–127 CrossRef CAS.
- Q. Q. Wang, S. H. Xu and F. L. Shen, Appl. Surf. Sci., 2011, 257(17), 7671–7677 CrossRef CAS.
- C. Wen, H. Deng, J. Y. Tian and J. M. Zhang, Trans. Nonferrous Met. Soc. China, 2006, 16, s728–s731 CrossRef.
- L. Elsellami, H. Lachheb and A. Houas, Mater. Sci. Semicond. Process., 2015, 36, 103–114 CrossRef CAS.
- Z. D. Zhu, Z. X. Chang and L. Kevan, J. Phys. Chem. B, 1999, 103, 2680–2688 CrossRef CAS.
- M. Hirano, C. Nakahara, K. Ota and M. Inagaki, J. Am. Ceram. Soc., 2002, 85(5), 1333–1335 CrossRef CAS.
- D. P. Xu, L. J. Feng and A. L. Lei, J. Colloid Interface Sci., 2009, 329(2), 395–403 CrossRef CAS PubMed.
- C. Kormann, D. W. Bahnemann and M. R. Hoffmann, Environ. Sci. Technol., 1991, 25(3), 494–500 CrossRef CAS.
- Y. Ku, R. M. Leu and K. C. Lee, Water Res., 1996, 30(11), 2569–2578 CrossRef CAS.
- E. Leyva, E. Moctezuma, M. G. Ruız and L. Torres-Martınez, Catal. Today, 1998, 40(4), 367–376 CrossRef CAS.
- A. Mills, R. H. Davies and D. Worsley, Chem. Soc. Rev., 1993, 22(6), 417–425 RSC.
- D. C. Schmelling, K. A. Gray and P. V. Kamat, Water Res., 1997, 31(6), 1439–1447 CrossRef CAS.
- N. Serpone, P. Maruthamuthu, P. Pichat, E. Pelizzetti and H. Hidaka, J. Photochem. Photobiol., A, 1995, 85(3), 247–255 CrossRef CAS.
- U. Stafford, K. A. Gray and P. V. Kamat, J. Phys. Chem., 1994, 98(25), 6343–6351 CrossRef CAS.
- A. T. Nguyen and R. S. Juang, J. Environ. Manage., 2015, 147, 271–277 CrossRef CAS PubMed.
- R. A. Doong, C. H. Chen, R. A. Maithreepala and S. M. Chang, Water Res., 2001, 35(12), 2873–2880 CrossRef CAS PubMed.
- K. S. Fancey, Vacuum, 1995, 46(7), 695–700 CrossRef CAS.
- C. M. Du, J. H. Yan and B. G. Cheron, Plasma Chem. Plasma Process., 2007, 27(5), 635–646 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02807a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.