Improving the photoelectrocatalytic performance of boron-modified TiO2/Ti sol–gel-based electrodes for glycerol oxidation under visible illumination†
Abstract
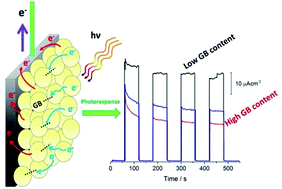
The effect of preparation variables such as the amount of water, the number of layers and the heating rate on the photoelectrocatalytic properties of boron-modified TiO2 sol–gel thin films was investigated. Materials were prepared by the sol–gel method and the deposition of a B-TiO2 film on Ti substrates by the dip-coating technique. The surface and chemical environment of the films was characterized by FESEM, DRS, XPS, and Raman spectroscopy and their photoelectrochemical properties were studied by voltammetry and photocurrent transient measurements under visible light. FESEM micrographs exhibit a cracked surface as a result of the increase of the capillary pressure in the films during drying to increase the number of layers. The Raman spectra show that the anatase phase was obtained during thermal treatment at 450 °C. Films employed as photoelectrodes in a three-electrode cell generated a higher photocurrent than the TiO2 film without modification did due to the presence of boron, which favors the formation of Ti3+ donor states identified by XPS, photogenerating more electrons in the semiconductor and promoting their transport to the substrate. The donor state density was estimated by Mott–Schottky plots. The highest photoresponse was obtained using a one-layer B-TiO2 film. In the presence of 1 M glycerol at pH 1, the photocurrent generated by the B-TiO2 films was increased about twice compared with the photocurrent observed in the absence of the organic, as a result of electron injection to the conduction band of TiO2 from the molecule during its oxidation in the film/electrolyte solution interface, promoting the current doubling effect.

 Please wait while we load your content...
Please wait while we load your content...