High transparency and toughness PMMA nanocomposites toughened by self-assembled 3D loofah-like gel networks: fabrication, mechanism, and insight into the in situ polymerization process
Huiwen He†
,
Si Chen†*,
Jun Bai,
Haiming Zheng,
Bozhen Wu,
Meng Ma,
Yanqin Shi and
Xu Wang*
College of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: wangxu@zjut.edu.cn; sichen@zjut.edu.cn; Fax: +86-0571-88320855; Tel: +86-0571-88320855
First published on 22nd March 2016
Abstract
Novel types of transparent PMMA composites were toughened with 3D loofah-like gel networks obtained via the in situ polymerization of methyl methacrylate (MMA) gel with POSS-based supramolecular POSS-Lys(BOC) gelators that have excellent compatibility with the polymerized matrix for the nanoscale nature of supramolecular gel fibers. The “gel networks-energy dissipation” toughening mechanism was investigated using SEM, tensile testing and DMA, the results of which demonstrate that a favorable architecture formed by the gel nanofibres through hydrogen bonding interactions contributes to the toughness improvement in these nanocomposites. In particular, UV-Vis spectrometry and haze meter tests indicate that the PMMA nanocomposites maintain the advantage of good optical transparency. In addition, the physical self-assembly nature of the gel networks allows them to be easily extracted from the polymer matrix, the result of which clearly demonstrates the framework formed by the nanofibres has not only been retained during the in situ polymerization process, but also has an outstanding capacity for the dissipation of energy. As a result, a special type of nanoporous materials with stable pore shapes was obtained, which has potential applications in optical sensors, energy storage and as a Li-ion battery separator.
Introduction
Poly(methyl methacrylate)1 (PMMA) is a commonly employed glassy thermoplastic polymer, commonly known as Plexiglas. Because of its excellent heat and cold resistance, corrosion resistance, good process abilities and excellent high transparency in the visible spectrum, PMMA is widely used in optical materials, biomedical materials, dental and surgical applications, optical communications and electronics, and porous materials.2 However, the applications of PMMA have been limited by its high brittleness. Many studies have been extensively carried out to improve the toughness of PMMA over the past several decades.3 Jansen and co-workers4 used rubber elastomers to toughen PMMA, while the tensile strength and light transmittance were significantly decreased because the system contained a lot of elastomer. Patra and co-workers5 modified PMMA with inorganic particles, but could not change the disadvantage in reduced light transmittance. Hong Dong and co-workers6 reported highly transparent and toughened PMMA using natural fibrillated cellulose nanofibrils (CNFs). In general, most of the polymer matrices are hydrophobic, whereas the CNFs are hydrophilic. Therefore, the preparation of cellulose-toughened PMMA was rather complicated. Unfortunately, it is still a great challenge to prepare modified PMMA materials with high toughness and high light transmittance using traditional methods.Well defined supramolecules have three-dimensional branched architectures with different structures in their molecular cores and constitute a unique nanoscale toolkit, which achieves a reversible sol–gel phase transition by means of the non-covalent nature of the interactions, including hydrogen bonding, π–π stacking, ion–ion, dipole–dipole, van der Waals forces, host–guest and ion coordination in the supramolecular network to form supramolecular gels.7 Recently, some research groups have paid their attention to applying self-assembling supramolecules in polymeric modification. Zubarev and co-workers8 prepared improved toughened polystyrene/gel network composites by the addition of bar-like gelators DRCs that assemble into a gel in the styrene monomer and then synthesized via in situ polymerization. Yang and co-workers9 took advantage of the 3D nanofiber network obtained from the self-assembly of DBS, which could gelate the liquid state precursor of polyurethane (SM6202). The obtained polyurethane nanocomposites containing a DBS supramolecular gel network showed higher toughness than the pure polymer while retaining their high luminousness. With the development of supramolecular science, the function of dendritic gelators was gradually exploited and applied in modifying PMMA by several researchers. Dendritic gelators, a special type of supermolecule, not only have the programmatically controllable and elemental information of supermolecular gelators, including change of chemical composition and regulation of topology, but also have a repetitive active group as polymer gelators providing multiple non-covalent interactions during the self-assembly process.10 Our group11 have proposed a facile strategy to reinforce PMMA using a 3D nanofibrillar gel network, which was formed from the self-assembly of dumbbell-liked dendritic gelators (G1-alkyl), only a small amount of gelator addition could significantly improve the mechanical properties of the obtained nanocomposites. However, although abovementioned studies shows outstanding improvement in the mechanical properties of the nanocomposites, the expression of the self-assembly of gelators and the true toughening mechanism haven't been verified to date.
In this study, POSS-Lys(BOC),12 a novel 3D radial symmetric organic/inorganic hybrid dendritic gelator, which can form a unique loofah-like network structure and macroscopically manifest itself with a high efficiency in gelation of MMA was synthesized and applied towards studying the toughening mechanisms in detail. After in situ UV polymerization of the MMA gels, the PMMA nanocomposites toughened by self-assembled nanofibrillar gel networks were successfully prepared. The molecular weight and molecular weight distribution, tensile strength, phase morphology, dynamic mechanical thermal properties and light transmittance performance of the obtained PMMA nanocomposites were investigated with gel permeation chromatography (GPC), scanning electron microscopy (SEM), dynamic mechanical thermal analysis (DMA), UV-Vis spectrometry and haze meter. It turns out that the gelator/PMMA composites exhibited significantly improved toughness with a 23% increase in the elongation at break, while retaining its high light transmittance of 92%. Moreover, the PMMA nanocomposites etched as a molecular imprinting porous material shows the conserved morphology self-assembled by the gelator during the in situ polymerization process, which further demonstrates the framework formed by nanofibres has an outstanding capacity for the dissipation of energy. This research expounds the toughening mechanism of an efficient approach via the in situ polymerization of supramolecular gel nanofibrillar networks to manufacture highly toughened PMMA composites with high light transmittance and shows the advanced application on porous materials using supramolecular gel templates. The results reported herein further demonstrate the potential of using self-assembled gel nanofibers to achieve enhanced toughness in high transparency polymer composites.
Experimental
Materials
Methyl methacrylate (MMA) (washed with caustic and distilled before use) was purchased from Shanghai chemical. Benzoin dimethyl ether (DMPA) was purchased from Aladdin (Shanghai, China).The gelator POSS-Lys(BOC) was designed and synthesized as reported in the literature.12 All solvents used in the synthesis were analytically pure and used without further purification.
Preparation of PMMA nanocomposites and etched PMMA nanocomposites
The process used to prepare the PMMA nanocomposites is shown in Scheme 2. A designed amount of gelator POSS-Lys(BOC) and photo-initiator DMPA (0.5 wt%) was added into MMA in a 20 mL glass cuvette. The mixture was sonicated for 30 min to break the large solid and then heated and shaken in an oil bath at ca. 120 °C until a transparent solution was obtained, as shown in step 1. Subsequently, the solution was poured into a mold, which was constructed of quartz glass, 1.0 mm in depth, 120 mm in width and 120 mm in length, and then allowed to cool to room temperature for 6 h to obtain stable gels, as shown in step 2. The stable gels formed were irradiated under a UV lamp (364 nm, 12 W) for 5 h, which was determined by a series of condition tests, as shown in step 3. The obtained materials containing gelators are denoted as PMMA nanocomposites. Depending on the content of POSS-Lys(BOC), the samples are denoted as 2% PMMA nanocomposites, or 5% PMMA nanocomposites (mass fraction).The prepared 2% PMMA nanocomposites were placed in a Soxhlet extractor with methanol and soaked overnight. Then, the nanocomposites were washed with hot methanol for 24 hours at 90 °C and finally evaporated in a vacuum drier for 2 hours at room temperature to obtain the etched PMMA nanocomposites, as shown in Scheme 2 in step 4.
Molecular weight and molecular weight distribution
The molecular weight and molecular weight distribution were determined on a Waters gel permeation chromatography (GPC) system at 80 °C. DMF was used as the eluent at a flow rate of 1.0 mL min−1 and the calibration of the molecular weight was carried out based on a poly(methyl methacrylate) standard.Measurement of the mechanical properties
The mechanical properties of the samples were measured using an electromechanical universal testing machine (CMT-4104, SANS) based on the test standard GB13022-91 (ISO527-3) and a resil impactor apparatus (CEAST, 9050). All the splines were tailed using a laser cutting machine (SCC1390, Wuhan Sangong). The dimensions of the izod notched impact splines were 80 × 10 × 1 mm3 and the dumbbell-shaped tensile splines were tailed based on the ASTM D638-10 standard.Dynamic mechanical analysis (DMA)
The DMA measurements were performed on a Dynamic Mechanical Analyzer (Q 800, TA) with a tensile mode at a frequency of 1 Hz. The scanning temperature range was from 40 °C to 160 °C at a scanning rate of 3 °C min−1. The sample size was 30 × 10 × 1 mm3.Field emission scanning electron microscopy (FE-SEM)
For the SEM image in Fig. 1a, a hot MMA solution of POSS-Lys(BOC) was allowed to cool to room temperature to form a stable gel and then dropped onto a silicon slide. The gel sample was evaporated in a vacuum drier at room temperature for 10 h to remove the ether solvent.For the SEM images shown in Fig. 4, the samples of three types of PMMA were impact spline sectioned and the sample of 2% PMMA nanocomposite first frozen by liquid nitrogen and then brittle ruptured. The fractured surfaces created were etched with methanol to remove the POSS-Lys(BOC). All the newly created fractured surfaces were coated with gold for FE-SEM (Hitachi S-4700, FEI) measurements. The accelerating voltage was 15 kV.
Transmission electron microscopy (TEM)
For the TEM image shown in Fig. 1b, the material as a MMA sol was drop coated on amorphous carbon-coated Cu grids and dried at room temperature. For Fig. 4f, the image of the PMMA nanocomposite was obtained from an ultra-thin section, which was cut from the plane perpendicular to the flow direction with a thickness of 75 nm. The TEM image was obtained using a JEM-1230 microscope at an accelerating voltage of 80 kV.Transmittance and haze of the PMMA composites
The transmittance and haze of the PMMA composites were measured using a haze meter in the visible region (WGT-S, Shanghai Changfang). The transmittance spectra were scanned over the range of 400–750 nm with a 6 nm interval, which was carried out in a UV-Vis spectrophotometer (Model Lambda 900).Results and discussion
Morphology of supramolecular gel networks
The morphology of the supramolecular gel networks, the so called 3D loofah-like network as we reported before,12 formed via a plane-to-plane stacking of the novel radial symmetric organic/inorganic hybrid gelator POSS-Lys(BOC) is shown in Fig. 1. It is clear from the images that the furcate fibers with a width of 50–300 nm meanders with no endpoints, they grow together inherently to form a continuous and integral network, without physical crosslinking of the individual fibers. That is the reason why POSS-Lys(BOC)/MMA gels have a strong resistance to external stress and exhibit a distinct viscous flow state. Taking advantages of such a unique organic/inorganic hybrid gelator may have unexpected effects in the toughening modification of PMMA.The influence of in situ polymerization on the molecular weight and molecular weight distribution
To study the influence of in situ polymerization with the import of gelators on the molecular weight and molecular weight distribution of the nanocomposites, the molecular weight and molecular weight distribution were tested through GPC. Table 1 shows that the number-average molecular weight (Mn) of the pure matrix and nanocomposites was 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, 27
000 g mol−1, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 24
000 g mol−1 and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, and the molecular weight polydispersity index (PDI) was 3.48, 4.58 and 4.49. From the results of in situ polymerization listed in Table 1, we can find out that during the in situ polymerization process, the nanostructure fiber network and MMA monomer are limited on their molecular level free motion for the “solid-like” gel-phase on its micro performance, which avoids the effects of the polymerization process of PMMA.13 Therefore, the molecular weight and molecular weight distribution of the PMMA nanocomposites and pure PMMA were of the same order of magnitude, which avoids any significant effect on the mechanical properties due to the addition of the gelators.14
000 g mol−1, and the molecular weight polydispersity index (PDI) was 3.48, 4.58 and 4.49. From the results of in situ polymerization listed in Table 1, we can find out that during the in situ polymerization process, the nanostructure fiber network and MMA monomer are limited on their molecular level free motion for the “solid-like” gel-phase on its micro performance, which avoids the effects of the polymerization process of PMMA.13 Therefore, the molecular weight and molecular weight distribution of the PMMA nanocomposites and pure PMMA were of the same order of magnitude, which avoids any significant effect on the mechanical properties due to the addition of the gelators.14
| Sample | DMPA (wt%) | POSS-Lys(BOC) (wt%) | Mn (g mol−1) | PDI |
|---|---|---|---|---|
| 1 | 0.5 | 0 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.48 |
| 2 | 0.5 | 2 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.58 |
| 3 | 0.5 | 5 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.49 |
Optical performance of the PMMA nanocomposites
There is no doubt that high transmittance is the most commendable feature of PMMA when compared with other polymeric materials. However, most PMMA composites are non-transparent due to the presence of reinforced fillers. Visually, these PMMA nanocomposites have high transparency, comparable to the pure PMMA matrix, as shown in Fig. 2. Fig. 2a displays the UV-Vis transmission spectra and digital images of pure PMMA and the PMMA nanocomposites with a thickness of 1 mm. The PMMA nanocomposites containing 2% upto 5% of gelators have similar transmittance of 92% and 91%, respectively, and that of pure PMMA of 96% in the visible wavelength region (550 nm). The average light transmittance and haze of pure PMMA and the PMMA nanocomposites were also tested using a haze meter, as shown in Fig. 2b. From the detailed data, the average value of the light transmittance measured from 400 to 700 nm (visible light region) was unobtrusive between pure PMMA and the PMMA nanocomposites of 92.8%, 93.4% and 92.9%. However, the haze of the PMMA nanocomposites was increased with an increase in the concentration of gelators from 0.6%, 4.5–7.7%, implying the loofah-like gel network has the capacity of light scattering. PMMA materials with high transmittance and haze have enormous potential applications in optical materials.15 Both types of PMMA nanocomposite shows relatively high light transmittance due to the nanoscale nature of the gelators and the excellent compatibility between the gels and PMMA matrix. Moreover, the loofah-like network has a capacity of light scattering. | ||
| Fig. 2 (a) The UV-Vis spectra of pure PMMA and the PMMA nanocomposites. (b) The average light transmittance and haze of pure PMMA and the PMMA nanocomposites. | ||
Mechanical properties and fracture surfaces
The mechanical properties of the PMMA composites were determined by considering their whole properties, tensile strength, breaking elongation and impact strength according to the experimental results. Fig. 3 shows the stress–strain curves for pure PMMA and the 2% and 5% PMMA nanocomposites. As shown in Fig. 3, pure PMMA without any fiber networks has a high tensile strength and low breaking elongation. The tensile strength of all the PMMA nanocomposites has the same value as pure PMMA for 53.9 MPa (0%), 53.5 MPa (2%) and 55.5 MPa (5%). However, their breaking elongation was increased from 4.9% to 5.4% and 5.9%. Simultaneously, all the samples have the same impact strength as pure PMMA for 4.4 kJ m−2, 4.4 kJ m−2 and 4.3 kJ m−2. From these results, we concluded that POSS-Lys(BOC) is a good toughening agent for the PMMA matrix within a low content and without reducing its tensile strength and impact strength.Although from the stress–strain diagram shown in Fig. 3, no clear evidence of changing the brittle nature of the PMMA via in situ modification could be observed; the quite different forms of the fracture surfaces shown in Fig. 4 indicate the nanoscale gel networks have the ability to dissipate load-energy. Fig. 4 shows the morphology of the fracture surfaces of pure PMMA and the 2% and 5% PMMA nanocomposites. Pure PMMA has a smooth fracture surface (Fig. 4a), indicating a typical brittle rupture. It was observed that the fracture surfaces of the PMMA nanocomposites (Fig. 4b and c) were rough and uneven, which can be attributed to partial adhesion when brittle rupture occurred. Moreover, there was a good compatibility between the gelators and the PMMA matrix because no phase interface was observed, which indicates the compositing effects of the PMMA and gelator are on a molecular level and avoids the adverse effects on the mechanical properties by the generation of a phase interface.
The prepared brittle failure 2% PMMA nanocomposite was placed in a Soxhlet extractor with methanol and soaked overnight to remove the POSS-Lys(Boc), the etched surface of which are shown in Fig. 4d and e. The POSS-Lys(Boc) can easily dissolve in methanol while PMMA cannot, and the etched surface shows the molecular imprinting of POSS-Lys(Boc) in the PMMA matrix. As shown in Fig. 4d, a large number of pores with a diameter of 50–300 nm are distributed on the surface and fracture surface of the membrane. In addition, from the fracture surface (Fig. 4e) a large number of 200–300 nm wide trenches of molecular imprinting as arrows referred are observed, which was attributed to the presence of loofah-like nanofiber networks. The distribution of the nanofiber networks was also determined by TEM as shown in Fig. 4f. Standing and lying fibres with a diameter of 50–300 nm are uniformly distributed in the matrix, which fit the pores and trenches, as shown in Fig. 4d and e. The diameter of the pores and the width of the trench fit the diameter of the nanofibers, about 50–300 nm, confirming the morphology of POSS-Lys(Boc) self-assembly in MMA was completely preserved and further demonstrated that the architectural network was not damaged during the in situ polymerization process. The morphology of the etched surface clearly shows the presence of the self-assembly behavior of the supramolecular gelator POSS-Lys(Boc) in the matrix and a positive toughening effect with the existence of an assembled fiber network in the PMMA.
The methanol solution after etching was also analyzed by time of flight mass spectrometry (TOF-MS) and the result is shown in Fig. 5. The result showed a peak at 1755.5 and the calculated [M + 2H]2+/2 of POSS-Lys(Boc) is 1755.0. The result fits with the calculated value perfectly, which shows that the POSS-Lys(Boc) was etched out without changing the chemical structure. These two results reveal that the chemical structure of the gelator was not changed during in situ polymerization of PMMA and the supermolecule gel structure was also preserved in PMMA by intermolecular forces, which further proves the inset network has the ability for the dissipation of energy and reduces the brittleness of PMMA. In addition, the nano-porous polymer materials manufactured using such a gel template leaching method16 have huge prospective applications in optical sensors, energy storage and as a Li-ion battery separator (Scheme 1).17
DMA is generally recognized to be sensitive to molecular motions and useful for evaluating the phase separation occurring in a polymeric system. The dynamic storage modulus (E′) is the most important property used to assess the load-bearing capability of materials and to characterize the stiffness of a material.18 The mechanical loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), the ratio of storage modulus and loss modulus, is a measure of the balance between the stiffness and toughness. Fig. 6a shows the influence of temperature on tan
δ), the ratio of storage modulus and loss modulus, is a measure of the balance between the stiffness and toughness. Fig. 6a shows the influence of temperature on tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of pure PMMA and the PMMA composites. Subsequently, the E′ of pure PMMA and the PMMA nanocomposites as a function of temperature is shown in Fig. 6b. In the range of measured temperature, E′ exhibits a decreasing tendency with an increase in temperature.
δ of pure PMMA and the PMMA composites. Subsequently, the E′ of pure PMMA and the PMMA nanocomposites as a function of temperature is shown in Fig. 6b. In the range of measured temperature, E′ exhibits a decreasing tendency with an increase in temperature.
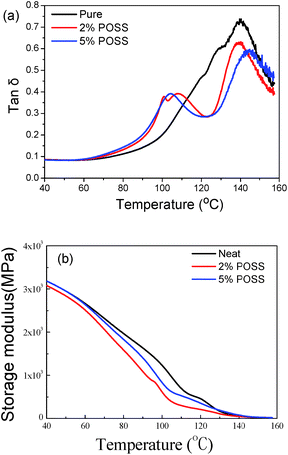 | ||
Fig. 6 tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and storage modulus as a function of temperature for pure PMMA and the PMMA nanocomposites. δ and storage modulus as a function of temperature for pure PMMA and the PMMA nanocomposites. | ||
From the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ-temperature curves, the maximum value of tan
δ-temperature curves, the maximum value of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ for pure PMMA was much higher than that found for both the 2% and 5% PMMA nanocomposites due to its amorphous structure and relatively free motion of molecular chains.19 In the presence of the gel network, the motion of the molecular chains is restricted, leading to low internal friction and low tan
δ for pure PMMA was much higher than that found for both the 2% and 5% PMMA nanocomposites due to its amorphous structure and relatively free motion of molecular chains.19 In the presence of the gel network, the motion of the molecular chains is restricted, leading to low internal friction and low tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values.20 According to the definition of loss factor, which implies that the material has certain damping capabilities. In general, the corresponding temperature of the maximum tan
δ values.20 According to the definition of loss factor, which implies that the material has certain damping capabilities. In general, the corresponding temperature of the maximum tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ can be defined as the glass transition temperature (Tg), which represents the transition temperature for the transition between the glass state and the viscoelastic state. From Fig. 6a, the PMMA nanocomposites have almost the same Tg at around 140 °C. In the 2% PMMA and 5% PMMA nanocomposites, a second transition of the shoulder peak occurred at ca. 100 °C. From the viewpoint of supramolecular chemistry, POSS-Lys(BOC) can self-assemble into loofah-like aggregates in a multi-stage process. These aggregates also disassemble at a certain temperature in a multi-stage process.21 The inclusion of network aggregates can act as restrained sites for crack initiation, which leads to toughness failure and improves the polymer performance.
δ can be defined as the glass transition temperature (Tg), which represents the transition temperature for the transition between the glass state and the viscoelastic state. From Fig. 6a, the PMMA nanocomposites have almost the same Tg at around 140 °C. In the 2% PMMA and 5% PMMA nanocomposites, a second transition of the shoulder peak occurred at ca. 100 °C. From the viewpoint of supramolecular chemistry, POSS-Lys(BOC) can self-assemble into loofah-like aggregates in a multi-stage process. These aggregates also disassemble at a certain temperature in a multi-stage process.21 The inclusion of network aggregates can act as restrained sites for crack initiation, which leads to toughness failure and improves the polymer performance.
From the E′–temperature curves, the two PMMA composites have lower storage modulus values, which reveals that the in situ formed POSS-Lys(BOC) supramolecular nanofibers can easily dissipate of energy and decrease the brittleness of PMMA. The results obtained from DMA demonstrating the supramolecular gel network can significantly improve the toughness of PMMA nanocomposites at routine temperatures.
“Gel networks-energy dissipation” toughening mechanism
In this study, the in situ polymerized PMMA nanocomposites have no difference in molecular weight in the obtained matrix and preserve the architecture-nanofibers network self-assembled by the supramolecular gelators. The framework formed via hydrogen bonding of the gel nanofibers in the polymer matrix provide effective energy dissipation under load, which prevents premature failure leading to strain and thus toughness improvement defined as a “gel networks-energy dissipation” toughening mechanism, as shown in Scheme 3. Scheme 3 shows the breaking progress simulation of the PMMA nanocomposites with the axial application of force, the framework dissipating the loading energy by deformation and collapsing of fiber networks. The dissipation of energy by fiber networks prompted the elongation at break of PMMA nanocomposites with a 23% increase, so as to improve the toughness of PMMA. However, the toughening of PMMA in this study behaves just like the brittle–ductile transition accompanied by decreased tensile strength. This is probably due to the use of interfacial hydrogen bonding interactions between the nanofibers, which are relatively weak when compared with covalent bonding and strong physical interactions. Based on understanding the mechanism in detail, we expect that the toughness of the PMMA nanocomposites could be further improved without decreasing the ultimate strength and modulus by utilizing highly effective supramolecules gel nanofibers. The results reported herein demonstrate the potential of using self-assembled gel nanofibers to achieve enhanced toughness in highly transparent polymer composites.Conclusions
An efficient solution to manufacture PMMA composites with both higher toughness and high transparency based on loofah-like 3D networks is reported using the in situ UV polymerization of MMA. The elongation at break, SEM, DMA, UV-Vis transmission spectra clearly show that the PMMA nanocomposites exhibited improved toughness when compared to pure PMMA with a retained high light transmittance. This can be attributed to the nanoscale nature of the supramolecule gel fibres and the excellent compatibility between the supramolecular gel fibres and the polymerized matrix formed in which the framework formed by the nano-fibres via hydrogen bonding in the polymer matrix provide effective energy dissipation under loading, preventing premature failure leading to strain. Thus, a toughness improvement act as “gel networks-energy dissipation” toughening mechanism is exposited. In addition, the nano-porous materials prepared with the technology of gel-template-leaching preserve the whole architectures and properties of the supramolecules, which provide a ready means of creating porous polymers applied in optical sensors, energy storage, Li-ion battery separators, and other fields by varying the concentration and structure of gel template.Acknowledgements
Financial support from the National Natural Science Foundation of China (Grant No. 21004052, 51173167), the Zhejiang Provincial Natural Science Foundation of China (Grant No. LY14E030003, LY14E030004) and Science and Technology Innovation Plan for College Students in Zhejiang Province (Xinmiao Talents Program: 2015R403035) is gratefully acknowledged.Notes and references
- M. P. Scott, M. Rahman and C. S. Brazel, Eur. Polym. J., 2003, 39, 1947 CrossRef CAS; F. J. Gómez, M. Elices and J. Planas, Eng. Fract. Mech., 2005, 72, 1268 CrossRef; M. H. Kim, J. H. Kim and C. K. Kim, et al., J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 2950 CrossRef.
- Y. Xia, M. Mrksich and E. Kim, et al., J. Am. Chem. Soc., 1995, 117, 9576 CrossRef CAS; J. A. Foster, D. W. Johnson and M. O. M. Pipenbrock, et al., New J. Chem., 2014, 38, 927 RSC.
- M. E. Fowler, H. Keskkula and D. R. Paul, Polymer, 1987, 28, 1703 CrossRef CAS; X. L. Xie, R. K. Y. Li and Q. X. Liu, et al., Polymer, 2004, 45, 2793 CrossRef; K. Cho, J. H. Yang and C. E. Park, Polymer, 1998, 39, 3073 CrossRef; K. Cho, J. H. Yang and C. E. Park, Polymer, 1997, 38, 5161 CrossRef.
- B. J. P. Jansen, S. Rastogi and H. E. H. Meijer, et al., Macromolecules, 2001, 34, 3998 CrossRef CAS; B. J. P. Jansen, S. Rastogi and H. E. H. Meijer, et al., Macromolecules, 2001, 34, 4007 CrossRef; B. J. P. Jansen, S. Rastogi and H. E. H. Meijer, et al., Macromolecules, 1999, 32, 6290 CrossRef.
- V. K. Singh, M. K. Patra and M. Manoth, et al., New Carbon Mater., 2009, 24, 147 CrossRef CAS.
- H. Dong, Y. R. Sliozberg and J. F. Snyder, et al., ACS Appl. Mater. Interfaces, 2015, 7, 25464 CAS.
- V. C. Edelsztein, A. S. Mac Cormack and M. Ciarlantini, et al., Beilstein J. Org. Chem., 2013, 9, 1826 CrossRef CAS PubMed; S. Chen, G. D. Tang, B. Z. Wu, M. Ma and X. Wang, RSC Adv., 2015, 5, 35282 RSC; S. Chen, H. W. He and G. D. Tang, et al., RSC Adv., 2015, 5, 101437 RSC.
- J. C. Stendahl, E. R. Zubarev and M. S. Arnold, et al., Adv. Funct. Mater., 2005, 15, 487 CrossRef CAS; J. C. Stendahl, L. Li and E. R. Zubarev, et al., Adv. Mater., 2002, 14, 1540 CrossRef; E. R. Zubarev, M. U. Pralle and E. D. Sone, et al., Adv. Mater., 2002, 14, 198 CrossRef.
- L. Jin, H. Wang and Y. Yang, Compos. Sci. Technol., 2013, 79, 58 CrossRef CAS.
- A. R. Hirst and D. K. Smith, Dendritic gelators, Springer, Berlin Heidelberg, 2005, p. 237 RSC; X. Yang, R. Lu and F. Gai, et al., Chem. Commun., 2010, 46, 1088 RSC; A. R. Hirst, D. K. Smith and M. C. Feiters, et al., J. Am. Chem. Soc., 2003, 125, 9010 CrossRef CAS PubMed; H. W. He, S. Chen and X. Q. Tong, et al., Soft Matter, 2016, 12, 957 RSC.
- F. Ye, S. Chen and G. D. Tang, et al., Colloids Surf., A, 2015, 480, 1 CrossRef CAS.
- G. D. Tang, S. Chen, F. Ye and X. Wang, Chem. Commun., 2014, 50, 7180 RSC.
- M. Geroge and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489 CrossRef PubMed.
- M. Takfuji, Y. Kira and A. Ishiordori, et al., Macromol. Symp., 2010, 291, 330 CrossRef.
- J. C. Boyer, N. J. J. Johnson and F. V. Veggel, Chem. Mater., 2009, 21, 2010 CrossRef CAS; G. H. Kim, Eur. Polym. J., 2005, 41, 1729 CrossRef; A. Tagaya, H. Ohkita and M. Mukoh, et al., Science, 2003, 301, 812 CrossRef PubMed.
- H. Gankema, M. A. Hempenius and M. Moller, et al., Macromol. Symp., 1996, 102, 381 CrossRef CAS; U. Beginn, Adv. Mater., 1998, 10, 1391 CrossRef.
- X. J. Fu, Y. Yang and N. X. Wang, et al., J. Mol. Recognit., 2007, 20, 238 CrossRef CAS PubMed; Y. Sun, L. Jin and H. Wang, et al., Soft Matter, 2011, 7, 348 RSC.
- Z. Jin, K. P. Pramoda and G. Xu, et al., Chem. Phys. Lett., 2001, 337, 43 CrossRef CAS; A. K. Saha, S. Das, D. Bhatta and B. C. Mitra, J. Appl. Polym. Sci., 1999, 71, 1505 CrossRef.
- G. S. Tay, T. Nanbo, H. Hatakeyama and T. Hatakeyama, Thermochim. Acta, 2011, 525, 190 CrossRef CAS.
- B. Harris, O. G. Braddell, D. P. Almond, C. Lefebvre and J. Verbist, J. Mater. Sci., 1993, 28, 3353 CrossRef CAS; J. L. Thomason, Polymer Composition, 1990, 11, 105 CrossRef.
- M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489 CrossRef CAS PubMed.
Footnote |
| † Huiwen He and Si Chen contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |







