Voltammetric determination of 4-chlorophenol using multiwall carbon nanotube/gold nanoparticle nanocomposite modified glassy carbon electrodes†
Abstract
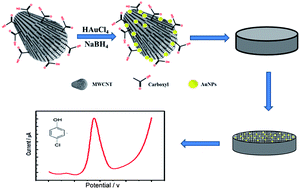
Carbon nanotubes (CNTs) have been employed as supporting materials for the dispersion of gold nanoparticles (AuNPs) to achieve improved catalytic properties. However, the application of CNT–AuNP composites for the evaluation of chlorophenols (CPs) is not reported. Herein, gold nanoparticle/carboxyl functionalized multi-walled carbon nanotube (AuNPs@cMWCNT) nanocomposites were synthesized via an in situ reduction method and further characterized with field emission scanning electron microscopy (FESEM), ultraviolet-visible (UV-vis) spectroscopy and X-ray diffraction (XRD). The AuNPs@cMWCNT modified glassy carbon electrode was subsequently prepared as a sensor for the electrochemical detection of 4-chlorophenol (4-CP). The electro-oxidation of 4-CP on the sensor is an adsorption-controlled irreversible process, which undergoes a two-electron and two-proton transfer. Under the optimal conditions, the oxidation peak current was proportional to the 4-CP concentration in the range of 0.3 to 400 μM, with a detection limit of 0.11 μM. The experimental data demonstrate that the sensor has great reproducibility, long-term stability, high specificity and excellent feasibility. The AuNPs@cMWCNT based sensor could be a facile, low-cost and rapid tool for the potential on-line monitoring of 4-CP in practical applications.

 Please wait while we load your content...
Please wait while we load your content...