Synthesis and characterization of chitosan–zinc composite electrodeposits with enhanced antibacterial properties
Xiaofan Zhaia,
Congtao Suna,
Ke Liab,
Fang Guanab,
Xueqing Liuc,
Jizhou Duan*a and
Baorong Houa
aKey Laboratory of Marine Environmental Corrosion and Bio-fouling, Institute of Oceanology, Chinese Academy of Sciences, No. 7 Nanhai Road, Qingdao 266071, China. E-mail: duanjz@qdio.ac.cn
bUniversity of Chinese Academy of Sciences, 19 (Jia) Yuquan Road, Beijing 100049, China
cQingdao National Oceanographic Center, No. 88 Xuzhou Road, Qingdao 266071, China
First published on 5th May 2016
Abstract
Zinc electrodeposits are one of the most popular safeguard procedures for steel constructions. However, in natural aquatic environments, especially marine environments, zinc electrodeposits suffer significantly from microbial induced corrosion and biofouling which lead to metal failure. To better confront the microbial induced problems, a biocide-zinc electrodeposit was synthesized based on chelating action. In this paper, nontoxic and biocidal natural polysaccharide, chitosan, was successfully incorporated into the zinc electrodeposit matrix, synthesizing a kind of chitosan–zinc composite electrodeposit. The addition of chitosan in the electrolyte influenced the surface morphology and crystalline structure of the resultant electrodeposits significantly, while a chitosan–zinc chelation complex was also found in the electrodeposits. A synthesis model was proposed in which a chitosan molecule could chelate zinc ions in the electrolyte by means of its N atoms in amino groups and O atoms in hydroxide radicals, which promoted the codeposition of zinc and chitosan during synthesis. Furthermore, remarkably enhanced broad-spectrum bactericidal properties of the chitosan–zinc electrodeposits were revealed through Escherichia coli, Pseudomonas aeruginosa and Shewanella oneidensis exposure. The best antibacterial properties of the resultant electrodeposits were obtained when the chitosan concentration was 0.6 g L−1 in the electrolyte.
1. Introduction
A zinc electrodeposit applied on a steel construction surface is one of the most extensively used techniques for providing sacrificial protection and barrier effects against corrosion at low cost.1–3 Various synthesis baths have been developed and employed in industry for zinc electrodeposits. The basic constituents of the baths are metal ions, conducting salts, buffers and additional agents. Because the additional agents and deposition conditions significantly influence the electrodeposit's properties,4,5 considerable interest has been generated for composite electrodeposits incorporating both inorganic and organic components within the zinc matrix.With respect to the inorganic functional components, researchers have found that enhanced corrosion resistance was obtained by alloying Al, Cr, and Mn6,7 as well as incorporating nanoparticles such as CeO2,8 TiO2,9 graphene oxide10 and halloysite nanotubes11 in zinc electrodeposits. Regarding to the organic ingredients, organic additives such as gelatin,12 trisodium nitrilotriacetic13 and ethylenediaminetetraacetate14 were also researched in electrolytes to obtain deposits with specific property. Furthermore, some organic–inorganic hybrid materials, such as carbon fiber reinforced plastic with zinc-coated steel15 and 5,5′-dimethylhydantoin with copper,16 were successfully synthesized for metals protection and barrier.17
The ultimate aim of these researches on electrodeposits with particular property was to make these electrodeposits applicable in natural environment. One of the most unsatisfactorily handled problems was considered to be the application of the zinc electrodeposits in moist environment accompanied by bacteria and fungi, especially the marine environment. In marine environment, microbiological induced corrosion and biofouling influenced the steel constructions seriously, leading to mechanical failure.18 In these processes, the attachment of bacteria plays an important role leading to the metal failure including the zinc electrodeposits break.19 To mitigate this severe problem, we've incorporated an organic biocide 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one20,21 into zinc electrodeposit, aiming at inhibiting the bacteria attachment.
On account of environment concerns, nontoxic organic components in metal electrodeposit should be developed. In this respect, as a natural polysaccharide with intrinsic antibacterial property, chitosan is considered to be a good choice. According to relevant reports,22,23 the antibacterial property of chitosan could be attributed to its cationic amino groups which interrupt the bacterial membrane and disrupt mass transport across bacterial cells. Chitosan has been applied to various biocidal composite films due to its natural, green and effective biocidal properties.24,25
As a result, chitosan was performed to be the organic component in the zinc composite electrodeposits for antibacterial properties in this study. The objective of this investigation was to synthesize and characterize chitosan–zinc composite electrodeposits, propose a reasonable synthesis model and then assess the antibacterial property by bacteria attachment research.
2. Experimental
2.1 Synthesis of the chitosan–zinc electrodeposits
A non-toxic sulfate zinc electrolyte, which is composed of 250 g L−1 ZnSO4·7H2O, 80 g L−1 Na2SO4, 26 g L−1 H3BO3 and 40 g L−1 Al2(SO4)3·18H2O, was used in this research. Pure zinc electrodeposit was deposited from this sulfate zinc electrolyte. To synthesis chitosan–zinc electrodeposits, chitosan added electrolyte were prepared by mixing gradient concentrations (0.2, 0.6 and 1.0 g L−1) of chitosan [(C6H11NO4)n, cat. no. C8320, Solarbio, China] into the sulfate zinc electrolyte. Analytical-grade reagents and distilled water were used to prepare high-purity solutions.Carbon steel (wt%: C 0.19, Si 0.31, Mn 0.55, P 0.04, S 0.043) specimens with dimensions of 50 mm × 13 mm × 2 mm were used as the electrodeposits substrates. Prior to deposition, steel specimens were ground with sandpaper to 2000# and oscillated by ultrasound in ethanol for 10 min. A carbon steel specimen was served as both the cathode and the working electrode during electrodeposition, whilst a pure zinc specimen of 50 mm × 20 mm × 5 mm was served as both the anode and the counter electrode. A saturated calomel electrode (SCE) was applied as the reference electrode connected by a salt bridge to the electrolyte. Under the control of a DJS-292E potentiostat, a current density of 5 mA cm−2 was utilized for synthesis and the deposition time was controlled to be 219 min. After deposition, the resultant coupons were washed with distilled water and dried.
The pure zinc electrodeposit obtained from sulfate zinc electrolyte was named C0, while the chitosan–zinc composite electrodeposits synthesized from 0.2, 0.6 and 1.0 g L−1 chitosan added electrolytes were named CCS1, CCS2 and CCS3, respectively. The mass of the coupon was measured before and after electrodeposition using an analytical balance to calculate the current efficiency, ηc, according to eqn (1):
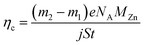 | (1) |
2.2 Characterization on the chitosan–zinc electrodeposits
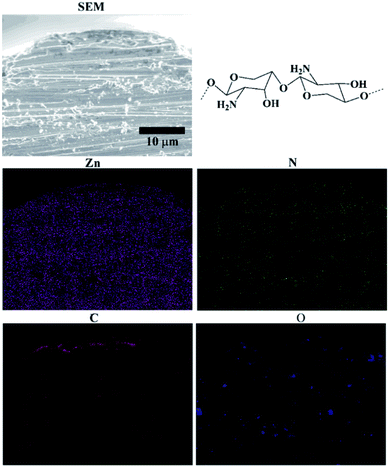
The phase structures of these electrodeposits were determined using an X-ray diffraction (XRD, Rigaku D/max-Ultima IV, Japan) under the following conditions: 40 kV, 30 mA, graphite-filtered Cu Kα radiation (l = 0.1542 nm). The electrodeposits morphologies and element distribution maps were analyzed using a scanning electron microscope (SEM, S-3400N, Hitachi, Japan) with an energy-dispersive spectrometer (EDS, INCAx, Oxford, UK) system. The molecule structure of chitosan in the composite electrodeposits was detected by a Fourier transform infrared spectroscopy (FT-IR, Nicolet iN10 IR Microscope, Thermo Fisher, US).2.3 Electrochemical analysis during synthesis process
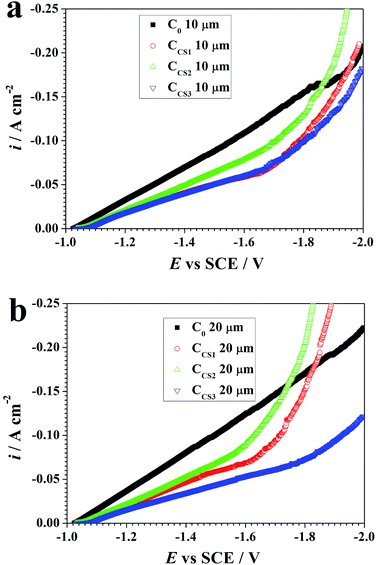
During the synthesis process, the depositing potential was monitored. The electrochemical impedance spectroscopy (EIS) and the cathodic potentiodynamic polarization curves were obtained when 10 μm and 20 μm electrodeposits were synthesized using a Solartron 1287/1260 electrochemical analyzer.A three-electrode system26 was applied with a 20 × 20 mm Pt specimen as the counter electrode and an SCE as the reference electrode connected by a salt bridge. EIS was performed when the 10 μm and 20 μm thick electrodeposits were synthesized. The impedance data was obtained at the depositing potential with the frequency interval analyzed from 100 kHz to 0.01 Hz. The width of the sinusoidal voltage signal applied to the system was 10 mV (rms). Cathodic potentiodynamic polarization curves were performed ranging from the open circuit potential to −200 mV versus SCE at a potential scanning rate of 5 mV s−1. The EIS data was analyzed using Princeton ZSimpWin version 3.21 software.
2.4 Antibacterial property assessment
Escherichia coli (E. coli) strain JM109 from Hong Kong University as well as Pseudomonas aeruginosa (P. aeruginosa) strain PAO-1 and Shewanella oneidensis (S. oneidensis) strain MR-1 from Zhejiang University isolated from marine environment were cultured by Luria-Bertani (LB) medium (pH adjusted to 7) containing 10 g L−1 NaCl, 10 g L−1 peptone from fish and 5 g L−1 yeast extract in distilled water. A bacterial colony on solid medium was inoculated into liquid LB medium and then cultured at 37 °C (for E. coli) and 30 °C (for P. aeruginosa and S. oneidensis), respectively, for 12 h. After culture, the concentrations of E. coli, P. aeruginosa and S. oneidensis in the medium were determined by colony-forming units (CFU).27Centrifugation at the speed of 4000 rpm for 5 min was performed to separate the bacterial body from the medium.28 Bacteria were then diluted and suspended in 0.1 mol L−1 phosphate buffer saline (PBS) (8.0 g L−1 NaCl, 0.2 g L−1 KCl, 1.44 g L−1 Na2HPO4, 0.44 g L−1 KH2PO4 in distilled water). PBS mediums containing 106 cfu mL−1 E. coli, P. aeruginosa and S. oneidensis were separately prepared for the immersion experiments.
The pure zinc electrodeposits and chitosan–zinc composite electrodeposits were then exposed in the 106 cfu mL−1 E. coli, P. aeruginosa and S. oneidensis PBS medium for 24 h, respectively. After exposure, the surfaces of the specimens were gently washed by sterile PBS and then immersed in PBS solution with 5% glutaraldehyde for 30 min. Subsequently the synthesized electrodeposits were dyed by 1 μg mL−1 4′,6-diamidino-2-phenylindole (DAPI) for 30 min. Fluorescent observation of bacteria was then performed by fluorescence microscopy (BX-51 with an image software of Cellsens, Olympus, Japan) at a magnification of 400×.
Analytical-grade reagents and distilled water were used to prepare LB and PBS media. All mediums were sterilized at 121 °C for 30 min before inoculation. Inoculation and system assembly were conducted in a sterile environment on an AIRTECH clean bench after 30 min ultraviolet light sterilization.
3. Results and discussion
3.1 Synthesis of the chitosan–zinc electrodeposits
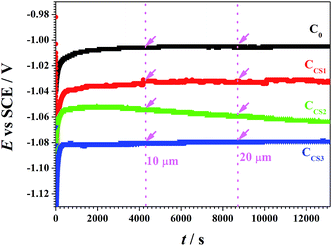
Fig. 2 displays that the depositing potential of C0 remained stable at −1.01 V vs. SCE, while the depositing potentials of CCS1, CCS2 and CCS3 reached −1.04 V, −1.06 V and −1.08 V vs. SCE, respectively. The apparent potential negative shifts during the chitosan–zinc synthesis process illustrated that chitosan molecule participated in the reducing reaction and adsorbed on the electrodepositing surface. Due to the absorption effect, chitosan molecule covered the electrodepositing surfaces, leading to a decrease of the effective surface area. So under continuous current, the depositing potential moved negatively. Therefore, chitosan effectively reduced the deposition potential, and higher concentration of chitosan revealed stronger influence.
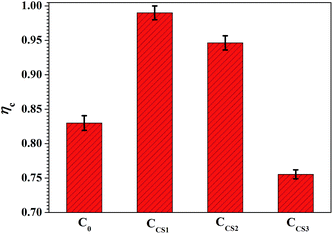
The pure zinc electrodeposit C0 revealed a normal current efficiency at 82.5%.21 The ηc of chitosan–zinc composite electrodeposits CCS1 and CCS2 reached relatively high values of 99.0% and 94.5%. On one hand, this phenomenon would be attributed to the high depositing potential (Fig. 2), which facilitated Zn2+ reduction; on the other hand, the inclusion of chitosan molecule inside the chitosan–zinc electrodeposits also led to the ηc increase. However, relatively high concentration of chitosan in electrolyte resulted in a decreased ηc of CCS3, which was attributed to the high absorption area of chitosan on the electrodepositing surfaces and the relative high reaction resistance (discussed in the latter section). Therefore, a proper concentration of chitosan addition into the electrolyte, in which CCS1 and CCS2 was synthesized, increased the current efficiency effectively.
3.2 Characterization of the chitosan–zinc electrodeposits
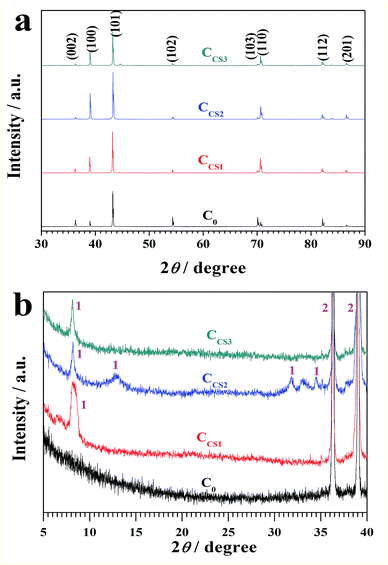 | ||
| Fig. 5 XRD results of the electrodeposits for zinc matrix (a) and chitosan–zinc chelation complex (b). | ||
To figure out the existence of the chitosan–zinc complex, a detailed XRD spectrum ranging from 5 to 40 degree is shown in Fig. 5b. Wang et al. synthesized pure chitosan–zinc chelation complex and analyzed its XRD results.33 On chitosan added zinc electrodeposits, especially CCS2, the main orientation peaks from chitosan–zinc chelation complex, which corresponded to Wang's reports, were found, proving the existence of chitosan–zinc chelation complex in the chitosan–zinc electrodeposits.
As a result, chitosan influenced the phase structure of the zinc matrix mainly by enhancing the (100) orientation and created a new chitosan–zinc complex phase. These influences were most obvious on CCS2 electrodeposit.
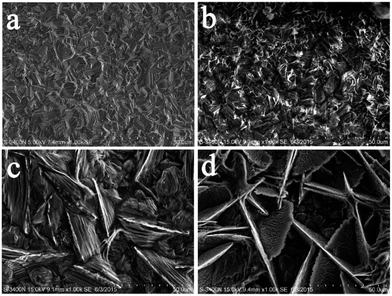
Since all the chitosan–zinc electrodeposits showed the same characteristic, only the EDS map of CCS1 is given in Fig. 7 to provide a visual result. Besides the main element Zn from the matrix, the typical elements C, N, and O from chitosan, emerged on the cross section. Furthermore, it could be found that C, N and O elements existed in all layers of the electrodeposit, indicating the successful chitosan incorporation inside the electrodeposit. Meanwhile, these elements appeared more in the surface layers than those in the inner layers, illustrating that more chitosan molecule were embedded in or absorbed on the surface layers than in the inner layers.
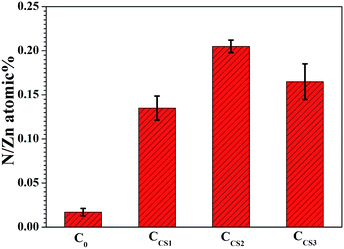
Nitrogen is one of the main elements constituting chitosan. As nitrogen is relatively stable and can be precisely detected by normal methods, the contents of chitosan in the electrodeposits were revealed by the N/Zn atomic ratio shown in Fig. 8. The data shown was calculated by EDS results and double checked by X-ray Photoelectron Spectroscopy measurements.
The N/Zn ratio of C0 was 0.015, approaching 0, revealing that hardly any nitrogen was found. Little nitrogen detected may be contributed to the N2 adsorbed on the samples. The N/Zn ratios of CCS1, CCS2 and CCS3 were calculated to be 0.14, 0.21 and 0.17, respectively, which is remarkably higher than that of C0. Among these three chitosan–zinc composite electrodeposits, CCS2 showed the highest N/Zn ratio, displaying the highest chitosan content.
By the EDS map research, chitosan was proved to be embedded inside the synthesized chitosan–zinc electrodeposits and gathered more in the surface layers. Furthermore, when the chitosan concentration was 0.6 g L−1 in the electrolyte, relatively high contents of chitosan in CCS2 was obtained.
3.3 Electrochemical synthesis model research
 | ||
| Fig. 9 The Nyquist plots of the electrodeposits during the electrodeposition process at 10 μm (a) and 20 μm (b). | ||
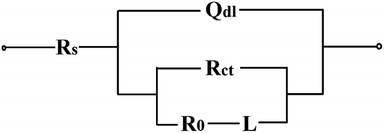
The circuit shown in Fig. 10 was applied to fit the EIS data. The main fitting parameters are displayed in Table 1.
| C0 | CCS1 | |||
|---|---|---|---|---|
| 10 μm | 20 μm | 10 μm | 20 μm | |
| Rs/ohm cm2 | 4.3 | 4.2 | 4.3 | 4.4 |
| Rct/ohm cm2 | 1.2 | 1.4 | 4.6 | 3.9 |
| CCS2 | CCS3 | |||
|---|---|---|---|---|
| 10 μm | 20 μm | 10 μm | 20 μm | |
| Rs/ohm cm2 | 3.9 | 4.5 | 4.5 | 4.1 |
| Rct/ohm cm2 | 4.1 | 4.5 | 5.5 | 4.9 |
As Table 1 shown, the solution resistance Rs values of the chitosan-added electrolytes were as same as that of the pure zinc electrolyte, which were approximately 4 ohm cm2. Rct represents the charge transfer resistance of the zinc reduction reaction. The Rct values of the pure zinc electrodeposit when 10 μm and 20 μm thick films synthesized shared the similar value of 1.2–1.4 ohm cm2. While Rct values of the chitosan–zinc electrodeposits when 10 μm thick films synthesized were 3.9–5.5 ohm cm2, which were much higher than that of the pure zinc electrodeposit. They remained similar at 20 μm within 4.1–4.9 ohm cm2. The Rct values increased with the chitosan adding concentration. The high Rct values of the chitosan–zinc electrodeposits illustrated that the chitosan molecule participated in the zinc reduction process by its chelation and adsorption effect. The EIS results indicated that the addition of chitosan dramatically influenced the synthesis process.
Both EIS and polarization curve results shared the same conclusion that chitosan dramatically influenced the synthesis process by its interaction with zinc ion in the electrolyte, thus increased the reducing reaction resistance.
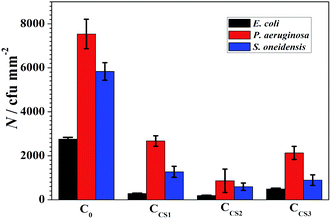
3.4 Antibacterial property of the chitosan–zinc electrodeposits
 | ||
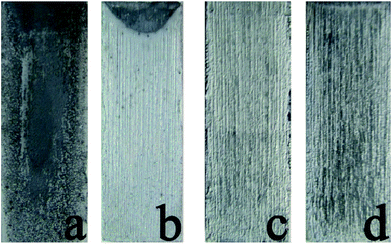
| Fig. 13 Fluorescence microscopy images of C0 (a), CCS1 (b), CCS2 (c) and CCS3 (d) after 24 h exposure in 106 cfu mL−1 E. coli PBS medium. | ||
After 24 h of exposure, large amounts of bacteria bodies attached on C0 surface, and many of them accumulated into multiple colonies, tending to form biofilm. By contrast, only a small amount of bacteria bodies got attached on the chitosan–zinc electrodeposits individually and dispersedly, illustrating an initial stage of attachment.
4. Conclusions
In summary, aiming at improving the properties of zinc electrodeposits applied in marine environment with aggressive microorganisms, chitosan–zinc composite electrodeposits with enhanced antibacterial properties against marine bacteria were successfully synthesized based on chelating action.As an organic additive in electrolyte, chitosan brightened the electrodeposits surfaces and effectively increased ηc by 16.5% at most. Meanwhile, chitosan obviously influenced the surfaced morphology and phase structure. Further FT-IR and XRD results showed that chitosan–zinc chelation complex was embedded inside the electrodeposits. Chitosan was also found to exist in the composite electrodeposits with effective structure. By further electrochemical analysis, chitosan increased the reaction resistance dramatically. Based on the electrochemical mechanism and characterization results, a synthesis model was proposed that chitosan molecule chelated zinc ion by the N atoms in amino groups and the O atoms in hydroxide radicals, which promoted the chitosan embedment in the zinc electrodeposits during the synthesizing process.
Antibacterial properties of the chitosan–zinc composite electrodeposits were analyzed through fluorescence microscopy observation in three kinds of bacteria suspended PBS medium over 24 h. The composite electrodeposits showed effectively enhanced broad-spectrum bactericidal properties. The CCS2 synthesized in electrolyte with the chitosan concentration of 0.6 g L−1 obtained the best antibacterial property with the bacteria attachment decreased by 90%. The resultant chitosan–zinc composite electrodeposits would supply a potential resolution for extending the service life of zinc electrodeposits on steel structures applied in microbial marine environments.
Acknowledgements
The present work was supported by the National Key Basic Research Project (No. 2014CB643304) and the Public Science and Technology Research Funds Projects of Ocean (No. 201405013-4).Notes and references
- V. Barranco, S. Feliu and S. Feliu, Corros. Sci., 2004, 46, 2203–2220 CrossRef CAS.
- Q. Li, Z. Feng, L. Liu, J. Sun, Y. Qu, F. Li and M. An, RSC Adv., 2015, 5, 12025–12033 RSC.
- Q. Li, Z. Feng, J. Zhang, P. Yang, F. Li and M. An, RSC Adv., 2014, 4, 52562–52570 RSC.
- K. O. Nayana and T. V. Venkatesha, J. Ind. Eng. Chem., 2015, 26, 107–115 CrossRef CAS.
- X. Ren, Y. Song, A. Liu, J. Zhang, P. Yang, J. Zhang, G. Yuan, M. An, H. Osgood and G. Wu, RSC Adv., 2015, 5, 64806–64813 RSC.
- Y. Wang and J. Zeng, Mater. Des., 2015, 69, 64–69 CrossRef CAS.
- A. E. Ares and L. M. Gassa, Corros. Sci., 2012, 59, 290–306 CrossRef CAS.
- R. A. Shakoor, R. Kahraman, U. S. Waware, Y. Wang and W. Gao, Mater. Des., 2014, 59, 421–429 CrossRef CAS.
- X. Yang, Q. Li, S. Zhang, F. Liu, S. Wang and H. Zhang, J. Alloys Compd., 2010, 495, 189–195 CrossRef CAS.
- R. Li, J. Liang, Y. Hou and Q. Chu, RSC Adv., 2015, 5, 60698–60707 RSC.
- M. Rajkumar, B. Devadas, S. M. Chen and P. C. Yeh, Colloids Surf., A, 2014, 4, 31230–31238 Search PubMed.
- Z. Xia, S. Yang and M. T. Tang, RSC Adv., 2014, 5, 2663–2668 RSC.
- M. F. de Carvalho and I. A. Carlos, Electrochim. Acta, 2013, 113, 229–239 CrossRef CAS.
- M. F. de Carvalho, E. P. Barbano and I. A. Carlos, Electrochim. Acta, 2013, 109, 798–808 CrossRef CAS.
- K. W. Jung, Y. Kawahito, M. Takahashi and S. Katayama, Mater. Des., 2013, 47, 179–188 CrossRef CAS.
- J. Zhang, A. Liu, X. Ren, J. Zhang, P. Yang and M. An, RSC Adv., 2014, 4, 38012 RSC.
- M. Barletta, A. Gisario, M. Puopolo and S. Vesco, Mater. Des., 2015, 69, 130–140 CrossRef CAS.
- J. Duan, S. Wu, X. Zhang, G. Huang, M. Du and B. Hou, Electrochim. Acta, 2008, 54, 22–28 CrossRef CAS.
- B. Little, P. Wagner and F. Mansfeld, Electrochim. Acta, 1992, 37, 2185–2194 CrossRef CAS.
- X. Zhai, X. Ma, M. Myamina, J. Duan and B. Hou, J. Solid State Electrochem., 2015, 19, 2213–2222 CrossRef CAS.
- X. Zhai, M. Myamina, J. Duan and B. Hou, Corros. Sci., 2013, 72, 99–107 CrossRef CAS.
- P. Li, Y. F. Poon, W. Li, H.-Y. Zhu, S. H. Yeap, Y. Cao, X. Qi, C. Zhou, M. Lamrani, R. W. Beuerman, E.-T. Kang, Y. Mu, C. M. Li, M. W. Chang, S. S. Jan Leong and M. B. Chan-Park, Nat. Mater., 2011, 10, 149–156 CrossRef CAS PubMed.
- P. Li, C. Zhou, S. Rayatpisheh, K. Ye, Y. F. Poon, P. T. Hammond, H. Duan and M. B. Chan-Park, Adv. Mater., 2012, 24, 4130–4137 CrossRef CAS PubMed.
- F. Wu, G. Meng, J. He, Y. Wu, F. Wu and Z. Gu, ACS Appl. Mater. Interfaces, 2014, 6, 10005–10013 CAS.
- Y. Zhang, L. Chen, C. Liu, X. Feng, L. Wei and L. Shao, Mater. Des., 2016, 92, 471–479 CrossRef CAS.
- L. Yu, J. Duan, W. Zhao, Y. Huang and B. Hou, Electrochim. Acta, 2011, 56, 9041–9047 CrossRef CAS.
- A. Bauer, W. Kirby, J. C. Sherris and M. Turck, Am. J. Clin. Pathol., 1966, 45, 493 CAS.
- Q. Chen, S. Ai, S. Li, J. Xu, H. Yin and Q. Ma, Electrochem. Commun., 2009, 11, 2233–2236 CrossRef CAS.
- R. Wiart, Electrochim. Acta, 1990, 35, 1587–1593 CrossRef CAS.
- K. O. Nayana and T. V. Venkatesha, J. Electroanal. Chem., 2011, 663, 98–107 CrossRef CAS.
- G. Meng, L. Zhang, Y. Shao, T. Zhang and F. Wang, Corros. Sci., 2009, 51, 1685–1689 CrossRef CAS.
- J. L. Ortiz-Aparicio, Y. Meas, G. Trejo, R. Ortega, T. W. Chapman and E. Chainet, J. Appl. Electrochem., 2012, 43, 289–300 CrossRef.
- X. Wang, Y. Du and H. Liu, Carbohydr. Polym., 2004, 56, 21–26 CrossRef CAS.
- Q. B. Zhang, Y. X. Hua, Y. T. Wang, H. J. Lu and X. Y. Zhang, Hydrometallurgy, 2009, 98, 291–297 CrossRef CAS.
- J. Bakker, A. Sanders and N. Van Rooijen, Biochim. Biophys. Acta, Biomembr., 1998, 1373, 93–100 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |