Synthesis and thermal energy storage properties of a solid–solid phase change material with a novel comb-polyurethane block copolymer structure
Xiaosheng Du,
Haibo Wang,
Xu Cheng and
Zongliang Du*
Textile Institute, College of Light Industry, Textile and Food Science Engineering, Sichuan University, Chengdu, 610065, China. E-mail: dzl407@163.com; Tel: +86-28-85401296
First published on 25th April 2016
Abstract
A novel phase change material (PCM), namely, comb polyurethane with polyethylene oxide segments as side chains (DMPEG–PU), was synthesized through the reaction between diethanolamine-modified monomethoxy polyethylene glycol (MPEG) with isophorone diisocyanate and 1,4-butanediol. The crystalline property, phase change property, and thermal stability were characterized by differential scanning calorimetry (DSC), thermogravimetric analysis (TG), wide-angle X-ray diffraction (WAXD), scanning electronic microscopy (SEM), and polarization optical microscopy (POM). An accelerated thermal cycling test was then conducted to reveal the thermal reliability of the synthesized DMPEG–PU. The WAXD patterns and POM images showed that the synthesized DMPEG–PU possesses a completely crystalline structure and smaller spherulites compared with the pristine MPEG. The DSC results showed that DMPEG–PU (with 85% weight percentage of MPEG) is a typical solid–solid PCM with high thermal transition enthalpy of more than 120 J g−1, which is higher than the thermal transition enthalpy of polyethylene glycol-based polyurethane. TG results showed that the synthesized DMPEG–PU exhibits satisfactory thermal stability.
Introduction
With increasing greenhouse gas emissions into the atmosphere and a shortage of mineral resources, thermal energy storage with phase change materials (PCMs) has gained increased attention.1–3 PCMs can absorb and release a large amount of heat energy during phase change with small fluctuations.4,5Polyethylene glycol (PEG) is a promising phase change material for thermal energy storage because of its relatively high phase change enthalpy, wide range of transition temperature, non-toxicity, good biocompatibility, relative stability, and non-corrosiveness.6–9 However, PEG is a classic solid–liquid phase change substance and has to be sealed to prevent leakage, which restrict its industrial application in thermal energy storage.10–13 Several researchers prepared solid–solid PCMs by using PEG as a phase change ingredient. Polyethylene glycol-based polyurethane (PEG–PU) is one of the prospective solid–solid PCMs.14,15 PEG, as a phase change ingredient (soft segment), is covalently bonded to diisocyanate (hard segment) to keep the material in solid state after PEG melting.16,17 Meng et al.18 prepared a linear polyurethane solid–solid PCM (phase change enthalpy = 100 J g−1) by using 1,4-butanediol (BDO) as chain extender. Su et al.19 prepared a solid–solid PCM by using high-molecular-weight PEG as soft segment. The phase change enthalpy is lower than that of the pristine PEG. Cao et al.20 prepared a hyperbranched polyurethane solid–solid PCM, whose phase change enthalpy is more than 100 J g−1. Overall, high thermal energy storage and proper phase change temperature are of great importance in fabricating PCMs. However, the polymer aggregation structure is difficult to restrain, and the cross-link structure is easily formed. The chemical bonds between PEG and diisocyanate restrict the free movement of PEG, thereby hindering PEG agglomeration and crystallization.21,22
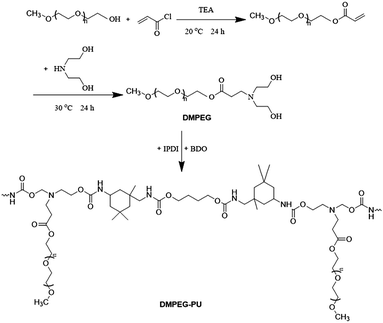
The arrangement and diethanolamine of polyethylene oxide segments are important factors that should be considered in increasing the phase change enthalpy of PEG–PU. In this work, a novel comb-polyurethane solid–solid PCM with polyethylene oxide as side chain was prepared by reacting calculated amounts of diethanolamine-modified monomethoxy polyethylene glycol (DMPEG) with isophorone diisocyanate and 1,4-butanediol (Fig. 1). While both ends of the PEG is linked to the main chain of polyurethane, only one end of the DMPEG is linked to the main chain of polyurethane, thereby activating the arrangement and orientation of DMPEG–PU. The properties of the synthesized PCM were investigated through differential scanning calorimetry (DSC), thermogravimetric analysis (TG), wide-angle X-ray diffraction (WAXD), scanning electronic microscopy (SEM), and polarization optical microscopy (POM). An accelerated thermal cycling test was conducted to determine the thermal stability of the synthesized PCM.
Experimental
Materials
MPEG and acryloyl chloride were purchased from Aladdin Reagent Co. Inc., America. Isophorone diisocyanate (IPDI) was purchased from Sigma-Aldrich Reagent Co. Inc., America. Polyethylene glycol (PEG, Mn = 5000 g mol−1; Shanghai Chemical Reagent Co. Inc., China) was degassed and dried in a round flask under high vacuum (20 Pa) at 100–120 °C for 3–4 h. Diethanolamine, triethylamine, BDO, and other common reagents were purchased from Chengdu Kelong Co. Ltd., China.Synthesis of diethanolamine modified monomethoxy polyethylene glycol
Monomethoxy polyethylene glycol ether acrylate (MPEGAC) was synthesized through the condensation reaction between MPEG and acryloyl chloride. Briefly, MPEG (20.00 g, 4 mmol), triethylamine (0.40 g, 4 mmol), and dichloromethane (DCM, 250 mL) were added to a flask at 0 °C. A DCM solution (30 mL) of acryloyl chloride (0.36 g, 4 mmol) was added dropwise. After 24 h, the solid salt was filtered and the filtrate was concentrated under reduced pressure. The crude product was purified three times by recrystallization in ethanol at −20 °C. MPEGAC was obtained by drying the precipitate under vacuum.DMPEG was synthesized by Michael addition between diethanolamine and MPEGAC. MPEGAC (20.36 g, 4 mmol), diethanolamine (1.68 g, 4 mmol), and ethanol (250 mL) were added to a flask at 30 °C. After 24 h, the final product was crystallized upon standing in a freezer overnight and the crude product was obtained through filtration. The crude product was purified three times by recrystallization in ethanol at −20 °C. DMPEG was obtained by drying the precipitate under vacuum.
Synthesis of DMPEG based polyurethane
DMPEG–PU was synthesized through two-step polymerization in a three-neck round bottomed flask fitted with an overhead stirrer. The synthesis route is shown in Fig. 1. A predetermined amount of dehydrated DMPEG (15.53 g, 3 mmol) and IPDI (2.14 g, 9.63 mmol) were mixed in freshly distilled butanone with stirring under nitrogen atmosphere at 75 °C for 2 h to prepare an NCO-terminated prepolymer. A predetermined amount of BDO (0.60 g, 6.63 mmol) was dissolved in butanone and slowly dropped into the stirring reaction mixture. After stirring for another 2.5 h at 75 °C, the reaction mixture was cast in a glass pan and placed in a vacuum oven at 60 °C for 48 h. The product was stored in vacuum at room temperature for 2 weeks before testing.PEG–PU was also prepared according to the synthesis route of DMPEG–PU for comparison.
Characterization
1H nuclear magnetic resonance (1H NMR) spectra of MPEGAC and DMPEG were recorded at 400 MHz by using a JEOL EX-400 spectrometer at room temperature with CDCl3 as the solvent.WAXD was employed to study the crystallization morphology by using D8 advance Bruker diffractometer. The incident X-ray was Cu Kα with 40 kV power and 30 mA and passed through a nickel filter. The WAXD curves ranging from 10° to 50° were collected at room temperature.
POM analysis was performed on a Leitz Laborlux 12POL microscope equipped with a video camera. The sample was placed between a microscope glass and a coverslip and then heated with a Leitz 350 hot stage.
Phase change temperature and enthalpy of PEG, PEG–PU, MPEG and DMPEG–PU were determined using differential scanning calorimeter (DSC 8500, Perkin-Elmer). Briefly, 5–10 mg of the samples were heated and cooled between −20 °C and 120 °C at a rate of 10 °C min−1 in nitrogen atmosphere. The sample was heated to 120 °C, kept at this temperature for 3 min, and cooled to −20 °C to eliminate the heat history of materials in the first scanning. In the second scanning, the sample was heated again to 120 °C, kept at 120 °C for 3 min, and then cooled to −20 °C. The second scanning was recorded.
Accelerated thermal cycling testing was conducted to confirm the thermal reliability of the synthesized PEG–PU and DMPEG–PU. Appropriate amount of the sample was placed in an aluminum pan and then loaded on a hot stage (STC200, Instec, USA) with a typical thermal cycling program that consisted of 100 times consecutive heating and cooling. The temperature was altered between 15 °C and 80 °C, and each thermal cycling was set to 20 min for a heating–cooling cycle.17 The phase transition properties of PEG–PU and DMPEG–PU after thermal cycling were studied by DSC analysis.
The thermal stability of the samples was characterized through thermogravimetric analysis using Netzsch STA409PC thermal analysis system. About 10 mg of the dried sample was weighed into an alumina crucible, and the curve was recorded from room temperature to 700 °C at a heating rate of 10 °C min−1.
SEM was used to study the phase morphology. Microphotographs were taken of the surface made by fracturing the specimen in liquid nitrogen and then casting it with gold (Au) powder. A HITACHI S-800 SEM was used for morphological observation.
Results and discussion
Synthesis of MPEGAC and DMPEG
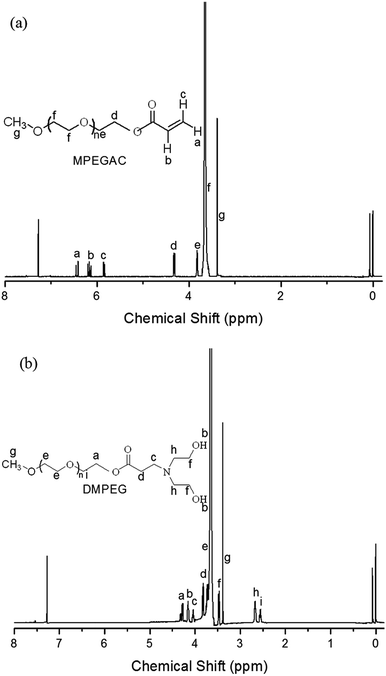
The detailed procedure for synthesis of MPEGAC and DMPEG is shown in Fig. 1. MPEGAC was synthesized by the condensation reaction between MPEG and acryloyl chloride. The chemical structure of MPEGAC was confirmed by 1H NMR analysis. As shown in Fig. 2(a), the ratio of the peak areas at 6.41, 6.15, and 5.86 ppm (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) as well as at 3.38 ppm (–OCH3) was 1
CH2) as well as at 3.38 ppm (–OCH3) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, indicating the successful synthesis of MPEGAC. DMPEG was synthesized by Michael addition between diethanolamine and MPEGAC. As shown in Fig. 2(b), the characteristic peaks between 5.80 and 6.50 ppm –CH
3, indicating the successful synthesis of MPEGAC. DMPEG was synthesized by Michael addition between diethanolamine and MPEGAC. As shown in Fig. 2(b), the characteristic peaks between 5.80 and 6.50 ppm –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 groups were not observed, indicating the successful Michael addition between –CH
CH2 groups were not observed, indicating the successful Michael addition between –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and diethanolamine. The 1H NMR spectrum of DMPEG indicated that the novel DMPEG was successfully synthesized.
CH2 and diethanolamine. The 1H NMR spectrum of DMPEG indicated that the novel DMPEG was successfully synthesized.
Crystalline properties investigation
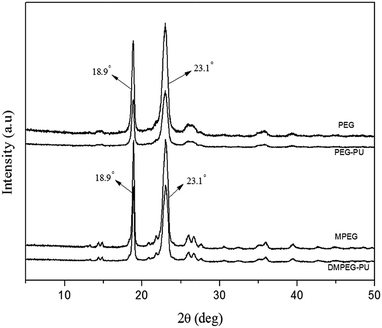
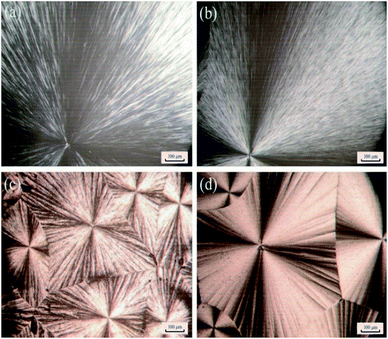
WAXD analysis was performed for the WAXD profiles to examine the crystallinity of PEG, PEG–PU, MPEG, and DMPEG–PU (Fig. 3). MPEG and DMPEG–PU exerted similar diffraction patterns, in which the diffraction angle and crystal plane distance were nearly the same. In the diffraction patterns of MPEG and DMPEG–PU, two strong diffraction peaks appeared at 18.9° and 23.1°. The result indicated that MPEG and DMPEG–PU possess the same crystal structure and unit cell type, and the crystal structure of MPEG is not influenced by preparation procedure. The diffraction peak height of DMPEG–PU is lower than that of pure MPEG, which indicated that the crystal particles of the former became smaller and the crystallinity decreased. Compared with PEG–PU, the diffraction peak height of DMPEG–PU is higher than that of PEG–PU, which indicated that DMPEG–PU obtained better crystallization than PEG–PU.The POM images of PEG, MPEG, PEG–PU and DMPEG–PU at room temperature are shown in Fig. 4 to directly observe the crystallization morphology. All samples showed obvious cross-extinction patterns, which suggested that all of them are crystalline and their crystalline morphologies are spherulites. However, spherulite size in PEG–PU and DMPEG–PU is smaller than that in PEG and MPEG, indicating that the arrangement and orientation of soft segments (polyethylene oxide) was limited by the hard segments in PEG–PU and DMPEG–PU. The crystallization process of PEG and MPEG became heterogeneous nucleation and confined crystallization, and crystallite size decreases.
Phase change properties
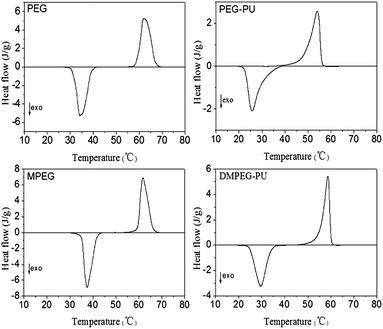
Fig. 5 shows the DSC curves of PEG, PEG–PU, MPEG, and DMPEG–PU, and Table 1 lists data on phase change temperature and enthalpy. All the four samples underwent phase transition with high transition enthalpy within 20–70 °C. The phase transition differs between PEG/MPEG and the synthesized PEG–PU/DMPEG–PU. As a typical solid–liquid PCM, PEG/MPEG transformed from a white crystal solid to a transparent liquid when the temperature was increased to about 62 °C. By contrast, PEG–PU and DMPEG–PU remained in solid state even when the temperature increased to 100 °C or higher. Hence, the synthesized material (DMPEG–PU) was found to be a solid–solid PCM with excellent thermal storage properties below 100 °C.| Sample | Phase transition | Theating (°C) | ΔHheating (J g−1) | Tcooling (°C) | ΔHcooling (J g−1) |
|---|---|---|---|---|---|
| PEG | Solid–liquid | 62.2 | 172.4 | 34.2 | 168.7 |
| PEG–PU | Solid–solid | 54.6 | 95.2 | 25.3 | 92.5 |
| MPEG | Solid–liquid | 61.9 | 171.4 | 37.5 | 168.1 |
| DMPEG–PU | Solid–solid | 59.0 | 121.9 | 29.1 | 118.1 |
As shown in Table 1, ΔHheating and ΔHcooling of PEG–PU and DMPEG–PU are lower than those of pristine PEG and MPEG, respectively; this finding indicated that phase change enthalpy weakened after PEG and MPEG reacted with diisocyanate. This result could be attributed to the limited free motion of PEG or MPEG when the end of the chain was fixed and to the compromised crystallization integrity of PEG.
The phase change enthalpy of DMPEG–PU was 121.9 J g−1 (ΔHheating), which was higher than that of PEG–PU (95.2 J g−1). This result can be attributed to the more active movement of polyethylene oxide segments in DMPEG–PU than that of PEG–PU. While both ends of the PEG was linked to the main chain of polyurethane, only one end of the DMPEG was linked to the main chain of polyurethane. Therefore, the arrangement and orientation of DMPEG–PU was more active; hence, DMPEG–PU could be an efficient thermal energy material with high phase change enthalpy and suitable phase change temperature.
Thermogravimetry analysis
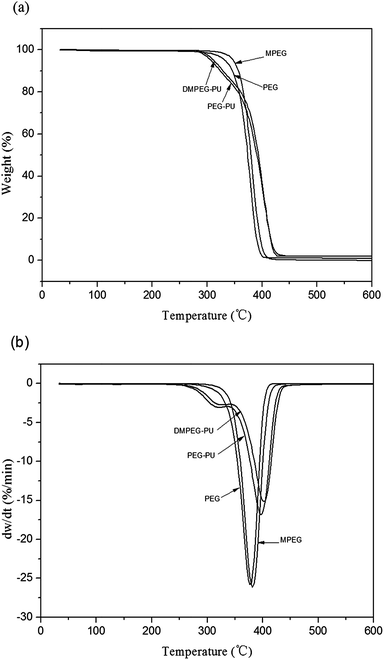
Thermogravimetry analysis was used to evaluate the thermal stability of PCMs. The obtained TG and derivative TG curves of PEG, PEG–PU, MPEG, and DMPEG–PU are shown in Fig. 6. As shown in Fig. 6(a) and (b), PEG and MPEG exhibited one-step thermal degradation behavior within 346–394 °C and 351–401 °C, respectively. The degradation of PEG–PU and DMPEG–PU were mainly consisted of two stages. The first decomposition temperatures of PEG–PU and DMPEG–PU were 288 °C and 293 °C, respectively. In this stage, mass loss was minimal. The mass loss of PEG–PU and DMPEG–PU were 11.3% and 11.9%, respectively. PEG–PU and DMPEG–PU were mainly degraded within 320–415 °C. The maximum degradation rates appeared at 397 °C and 401 °C for PEG–PU and DMPEG–PU, respectively. The main degradation temperature of PEG–PU and DMPEG–PU increased by about 15 °C compared with that of the pristine PEG and MPEG. The first step was decomposition step corresponding to the thermal degradation of urethane bonds in polyurethane, and the second step was attributed to the thermal degradation of the MPEG/PEG chains.23 These results implied that DMPEG–PU exhibited satisfactory thermal stability and therefore has broad applicable temperature range.Thermal reliability analysis
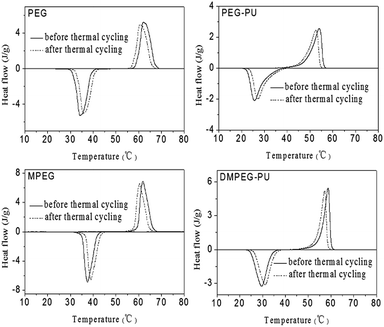
Thermal reliability is an important factor in phase change material research and applications because it affects the capacity for thermal storage. The DSC curves of the synthesized PEG, MPEG, PEG–PU and DMPEG–PU before and after 100 thermal cycling are shown in Fig. 7, and the phase change properties are listed in Table 2. The phase change temperatures and enthalpy of PEG–PU and DMPEG–PU minimally changed after 100 thermal cycles. The loss ratio of the phase change enthalpy for PEG–PU and DMPEG–PU were less than 2% after 100 thermal cycles, which is a good property for PCMs. These results indicated that PEG–PU and DMPEG–PU exhibited good thermal reliability and excellent reusability in terms of thermal energy storage and release properties.| Sample | Theating (°C) | ΔHheating (J g−1) | Tcooling (°C) | ΔHcooling (J g−1) |
|---|---|---|---|---|
| PEG | 61.2 | 169.8 | 35.1 | 166.1 |
| MPEG | 61.0 | 168.6 | 38.1 | 165.6 |
| PEG–PU | 53.5 | 93.8 | 26.3 | 91.1 |
| DMPEG–PU | 58.1 | 119.5 | 30.2 | 115.8 |
Scanning electronic microscopy analysis
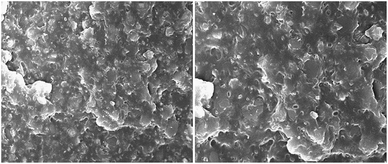
DMPEG–PU is a comb-polyurethane composed of the hard segment and the soft segment. The SEM photographs indicating the microstructure of DMPEG–PU and PEG–PU are shown in Fig. 8. From the figure, it can be observed that the soft segment, which is the black part, acting as working materials for the heat storage is dispersed into the hard segment, which is the white part, acting as the “physical cross-links”.19,24 The existence of the hard segment is negative for the soft segment's crystallization and transition enthalpy, but it plays a very important role in DMPEG–PU's solid state keeping ability. Since the hard segment domain, serving as “physical cross-links”, restricts the free movement of polyethylene oxides segments, when it is heated to a temperature 40 °C above MPEG's melting point, DMPEG–PU still does not move freely and keep its solid state. Viewing from the microcosmic level, in the process of DMPEG–PU's phase transition, with the temperature increasing, the soft segment MPEG's molecular thermal movement is quickening. It breaks away from the bondage of intermolecular forces. The crystalline perfection of MPEG has been destroyed. So, it turns into an amorphous state, but the amorphous MPEG is connected with the high melting temperature hard segment. It can only vibrate and rotate but can not translate freely. It shows a special solid–solid phase change behavior. Thus, it can be concluded that DMPEG–PU's phase transition is the soft segment MPEG's transition from a crystalline solid state to an amorphous solid state essentially. The energy is mainly obtained from the heat caused by the entropy change between the low entropy crystal state and the high entropy amorphous state.Conclusion
A novel comb-polyurethane solid–solid PCM with polyethylene oxide segments as side chain was successfully synthesized through bulk polymerization. While both ends of the PEG was linked to the main chain of polyurethane, only one end of the DMPEG was linked to the main chain of polyurethane, which activated the arrangement and orientation of DMPEG–PU more active. We conclude that DMPEG–PU with solid–solid phase change behavior of high thermal energy storage capability, suitable phase transition temperature, good thermal stability and reliability is a novel type of solid–solid phase change material for thermal energy storage and temperature control. Therefore, DMPEG–PU exerts great potential for thermal storage applications.Acknowledgements
This study was supported by the Natural Science Foundation of China (NSFC 51073101, 51503130), support plan of Science and Technology Department of Sichuan Province, China (No. 2012GZ0096) and Xiamen Southern Ocean Research Center Program (14GZP004NF04, 14GQT61HJ31).Notes and references
- X. Fang, L. Fan, Q. Ding, X. Yao, Y. Wu, J. Hou, X. Wang, J. Yu, G. Cheng and Y. Hu, Energy Convers. Manage., 2014, 80, 103–109 CrossRef CAS.
- F. He, X. Wang and D. Wu, Renewable Energy, 2015, 74, 689–698 CrossRef CAS.
- A. Mojiri, R. Taylor, E. Thomsen and G. Rosengarten, Renewable Sustainable Energy Rev., 2013, 28, 654–663 CrossRef.
- M. M. Farid, A. M. Khudhair, S. A. K. Razack and S. Al-Hallaj, Energy Convers. Manage., 2004, 45, 1597–1615 CrossRef CAS.
- J. Paris, M. Falardeau and C. Villeneuve, Energy Sources, 1993, 15, 85–93 CrossRef CAS.
- C. Chen, W. Liu, Z. Wang, K. Peng, W. Pan and Q. Xie, Sol. Energy Mater. Sol. Cells, 2015, 134, 80–88 CrossRef CAS.
- K. Chen, R. Liu, C. Zou, Q. Shao, Y. Lan and X. Cai, Sol. Energy Mater. Sol. Cells, 2014, 130, 466–473 CrossRef CAS.
- C. Alkan, E. Gunther, S. Hiebler, O. Ensari and D. Kahraman, J. Sol. Energy, 2012, 86, 1761–1769 CrossRef CAS.
- M. Jonsson, O. Nordin, E. Malmstrom and C. Hammer, Polymer, 2006, 47, 3315–3324 CrossRef CAS.
- Z. Zhang, G. Shi, S. Wang, X. Fang and X. Liu, Renewable Energy, 2013, 50, 670–675 CrossRef CAS.
- C. Chen, L. Wang and Y. Huang, Appl. Energy, 2011, 88, 3133–3139 CrossRef CAS.
- Y. Jiang, E. Ding and G. Li, Polymer, 2002, 43, 117–122 CrossRef CAS.
- P. Xi, L. Xia, P. Fei, D. Zhang and B. Cheng, Sol. Energy Mater. Sol. Cells, 2012, 102, 36–43 CrossRef CAS.
- C. Alkan, E. Gunther, S. Hiebler, Ö. F. Ensari and D. Kahraman, Sol. Energy, 2012, 86, 1761–1769 CrossRef CAS.
- P. Xi, X. Gu, B. Cheng and Y. Wang, Energy Convers. Manage., 2009, 50, 1522–1528 CrossRef CAS.
- P. Xi, L. Xia, P. Fei, D. Zhang and B. Cheng, Sol. Energy Mater. Sol. Cells, 2012, 102, 36–43 CrossRef CAS.
- C. Chen, W. Liu, H. Wang and K. Peng, Appl. Energy, 2015, 152, 198–206 CrossRef CAS.
- Q. Meng and J. Hu, Sol. Energy Mater. Sol. Cells, 2008, 92, 1260–1268 CrossRef CAS.
- J. Su and P. Liu, Energy Convers. Manage., 2006, 47, 3185–3191 CrossRef CAS.
- Q. Cao and P. Liu, Eur. Polym. J., 2006, 42, 2931–2939 CrossRef CAS.
- A. Sari, C. Alkan, A. Bicer and A. Karaipekli, Sol. Energy Mater. Sol. Cells, 2011, 95, 3195–3201 CrossRef CAS.
- A. Sari, C. Alkan and A. Bicer, Mater. Chem. Phys., 2012, 133, 87–94 CrossRef CAS.
- Z. Liu, X. Fu, L. Jiang, B. Wu, J. Wang and J. Lei, Sol. Energy Mater. Sol. Cells, 2016, 147, 177–184 CrossRef CAS.
- G. Ke, H. Xie, R. Ruan and W. Yu, Energy Convers. Manage., 2010, 51, 2294–2298 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |