Solvent-free catalytic synthesis and optical properties of super-hard phase ultrafine carbon nitride nanowires with abundant surface active sites
Jilin Wanga,
Lulu Zhanga,
Fei Long*a,
Weimin Wang*b,
Yunle Guc,
Shuyi Moa,
Zhengguang Zoua and
Zhengyi Fub
aSchool of Materials Science and Engineering, Key Laboratory of Nonferrous Materials and New Processing Technology of Ministry of Education, Guilin University of Technology, Guilin 541004, China. E-mail: longf@glut.edu.cn; jilinwang@glut.edu.cn; Fax: +86-773-5896436; Tel: +86-773-5896700
bThe State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China
cNano and Ceramic Materials Research Center, Wuhan Institute of Technology, Wuhan 430073, China
First published on 22nd February 2016
Abstract
High-quality ultrafine α/β-carbon nitride (α/β-C3N4) nanowires have been fabricated through a novel hot melt reduction synthetic method using polyvinylchloride ([–C2H3Cl–]n), ammonium chloride (NH4Cl) and ferric oxide (Fe2O3) as raw materials. The purity, structure, morphology, crystallinity and surface state of the as-prepared samples were investigated by FSEM, TEM, HRTEM, SAED, XRD, EDX, FTIR and XPS. The nanowires presented good crystallinity with a length range of 1–4 μm and an average diameter of about 10 nm. Every nanowire possessed a high specific surface area and rough surface with abundant exposed atoms/prominences, indicating that the surface structure will facilitate further surface modification, functionalization and related applications. In addition, UV-vis diffuse reflectance and the corresponding photoluminescence (PL) spectra indicated that the nanowires have a wide band gap (4.38 eV) and obvious ultraviolet luminescence properties at the maximum emission peak of about 340 nm. A catalytic reaction mechanism and the growth model were also proposed to explain the formation process of the C3N4 nanowires.
1. Introduction
Carbon nitride has been attracting more and more attention as a promising functional material, since Liu and Cohen predicted that β-C3N4 possessed a high level of hardness comparable to or even exceeding that of diamond.1,2 The theoretical calculations indicated that there were five crystal structures of C3N4, including α-C3N4, β-C3N4, cubic-C3N4, pseudocubic-C3N4 and graphitic C3N4. In these C3N4 phases, apart from graphitic C3N4, the other four are super-hard phase materials.3–6 As for the super-hard phase carbon nitride, they have many excellent physical and chemical properties, such as high hardness, low density and friction coefficient, good chemical inertness and thermal stability, are nonpoisonous, have wide band gap, and so on. These unique properties make super-hard phase carbon nitride suitable for a broad range of applications in the fields of mechanics, tribological and wear-resistant coatings (anti-scratch coatings), national defense, optical and electronic engineering, hydrogen storage, etc.1,2,7–15Several synthesis methods have been performed to fabricate these potentially super-hard phase carbon nitride materials, including physical vapor deposition,16–19 chemical vapor deposition,8,20,21 electrochemical deposition,22 solvothermal method,23–25 high temperature and pressure process26,27 and high energy ball milling reactions,28,29 etc. Among them, the former three deposition technologies were usually used to prepare super-hard phase carbon nitride films. However, most of the obtained films demonstrated low nitrogen content, changeful carbon to nitrogen ratios, amorphous and containing the other impurity elements. Even though previous research had reported the partially or wholly crystallized carbon nitride films, the results were only based on the XRD and TEM characterization, lacking reliability.30,31 On the other hand, as for the super-hard phase carbon nitride powders, high temperature and high pressure method required extreme conditions.27 High energy ball milling assisted with annealing processes was an effective approach to obtain α/β-C3N4 nano-materials, while the metal element (such as Fe) came from milling balls would react with C and formed difficultly removed compounds. Solvothermal method usually prepared graphite C3N4, α/β-C3N4 and/or the other multiple-phase carbon nitride structures.13,24,25,32
Generally speaking, on the basis of the literature analysis, it was obvious that the preparation and application research of super-hard phase carbon nitride materials mainly focused on the films. On the contrary, the corresponding research of super-hard phase carbon nitride powders processed at a slow pace up to now. It is due to the difficulty for effectively synthesis of high quality super-hard phase carbon nitride powders. Although few papers have reported the successful fabrication of different super-hard phase carbon nitride crystals or powders, the purity, yield and crystallinity of the products were unsatisfactory. Furthermore, the distinct and comprehensive chemical structure characterization was also difficult, limiting the related growth mechanism, physical and chemical properties, as well as applications of the super-hard phase carbon nitride. Therefore, it is urgent and significant to explore a practicable approach to achieve large amount of high quality super-hard phase carbon nitride powders.
In this paper, a novel kind of ultrafine α/β-C3N4 nanowires has been successfully fabricated through an effectively hot-melt reduction synthetic route for the first time. This method belongs to a solvent-free synthesis approach which is suitable for large amounts industrialized production. In addition, detailed characterizations have been carried out to determine the purity, structure, morphology, crystallinity and surface state of the as-prepared samples. On the base of the experimental results, the possible chemical reactions and growth mechanism were proposed to properly interpret the formation of the C3N4 nanowires. Besides, the band gap and optical property of the as-synthesized were also investigated.
2. Experimental
In a typical synthesis process, 2.38 g polyvinylchloride (PVC, [–C2H3Cl–]n, A.R.), 5.40 g ammonium chloride (NH4Cl, A.R.) and 1.59 g ferric oxide (Fe2O3, A.R.) were mixed and placed into a stainless autoclave of 30 ml capacity. The autoclave was sealed tightly and put into a vertical crucible furnace. Then the furnace was heated to 650 °C at a speed of 6 °C min−1 and maintained for 6 h. After that, the autoclave was cooled down to ambient temperature naturally and opened. The products were washed with hydrochloric acid, nitric acid, distilled water and anhydrous ethanol respectively. Finally, black powders were collected after desiccated in vacuum at 80 °C for 24 h.The structure of the as-synthesized samples was analyzed using an X-ray diffraction (XRD, Rigaku D/MAX-LLIA X-ray diffractometer with Cu-Kα radiation). The element composition, valence, and chemical bond types were characterized by X-ray energy dispersive spectroscopy (EDX) attached to FSEM FEI Quanta FEG 250, Fourier transform infrared spectra (FTIR, Nicolet Nexus) and X-ray photoelectron spectroscopy (XPS, VG Multilab 2000). The morphology and microstructure were studied with field scanning electron microscopy (FSEM, FEI Quanta FEG 250), transmission electron microscopy (TEM, JEOL JEM-2100F and Philip CM12), selected-area electron diffraction (SAED, Philip CM12). The UV-vis diffuse reflectance spectroscopy (DRS, Shimadzu, UV-3600) was performed using BaSO4 as the reference. The photoluminescence (PL) spectra were collected using a fluorescence spectrophotometer (Cary Eclipse VA, VARIAN).
3. Results and discussion
3.1. Results
Fig. 1(a) and (b) show the typical FSEM images with different magnifications of the as-prepared C3N4 samples. Large quantities of one dimension nanowires could be observed, presenting a length range of 1–4 μm and diameter range of 8–20 nm. It is worthy to note that some platelets and particles also exist in the samples (pointed out by the white frames). It is due to the residual amorphous carbon or the other byproducts formed during the growth process of the C3N4 nanowires. In addition, the purity of nanowires was estimated of higher than 85 wt%. Fig. 1(c) gives the typical EDX single nanowire line-scan spectra of the as-prepared C3N4 samples. The dominant elements C, N and O could be found and every element is distributed evenly. The O can be attributed to the surface oxidation and hydrolysis of the nanowires. Besides, quantitative analysis demonstrates that the molar ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N is 3
N is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.59, indicating high nitrogen content. The EDX results suggest that the obtained samples are one dimension carbon nitride compounds.
4.59, indicating high nitrogen content. The EDX results suggest that the obtained samples are one dimension carbon nitride compounds.
 | ||
| Fig. 1 Typical FSEM images (a and b) and EDX single nanowire line-scan spectra (c) of the as-synthesized C3N4 samples. Scale bars: (a) 2 μm and (b) 500 nm. | ||
Fig. 2 shows the typical TEM images of the as-synthesized C3N4 samples. It is obvious that the nanowires display solid internal structures with an average diameter of about 10 nm. Of course, some amorphous platelets and nanoparticles also be found in the high magnification TEM images (Fig. 2(b)–(d), marked by white dashed frames), which is well coincided with the above-mentioned FSEM analysis results. Moreover, it is interesting that the nanowires present a rough surface and continually growing tendency along the radial direction, as shown in the enlarged images of single nanowire (denoted by solid frames in Fig. 2(a) and (d)).
 | ||
| Fig. 2 Typical TEM images of the as-synthesized C3N4 samples. Scale bars: (a) 100 nm, (b) 50 nm, (c) 50 nm and (d) 50 nm. | ||
Fig. 3 displays the HRTEM images of the as-synthesized C3N4 samples. It can be seen that the nanowires present rough surface and clear lattice fringe with different interlayer spacings of about 0.202 nm (Fig. 3(a) and (b)) and 0.242 nm (Fig. 3(c) and (d)), which corresponds well to the (210) plane of β-C3N4 and (201) plane of α-C3N4, respectively. In addition, both α and β phase C3N4 nanowires have a diameter of about 10 nm but different angles (0°, 12.5°, 25° and 60°) between crystal face array and axial directions of α and β phase C3N4 nanowires. Maybe the angle has been determined at the beginning of the growth process of the single C3N4 nanowire, which should be further studied in the future.
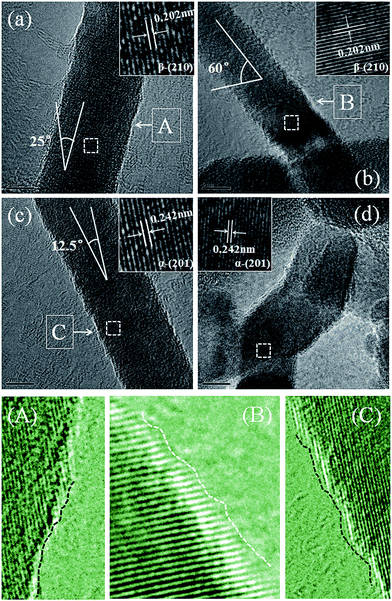 | ||
| Fig. 3 Typical HRTEM (a–d) and the corresponding enlarged images of the as-synthesized C3N4 samples. Scale bars: (a) 5 nm, (b) 5 nm, (c) 5 nm and (d) 5 nm. | ||
In order to make clear the surface condition of the C3N4 nanowires, the partial magnification HRTEM images have been investigated carefully (as shown in Fig. 3(A)–(C), pointed out by white arrows). The rough surface of nanowires is attributed to neither adhered other shaped byproduct particles nor surface breakage, but the different growth rates of the neighboring layers along the direction parallel to the crystal plane (marked with dashed lines). The crystal plane growth rate on one side is depended on the atom deposition arraying rate on this side, finally leading to the rough surface of the nanowires. Therefore, different growth angles and rough surface are both closely related with the growth mechanism of the different C3N4 nanowires. On the other hand, it is worth for special concern that every nanowire possesses a high specific surface area with abundant exposed atoms/prominences (marked with dashed lines). These exposed atoms/prominences will act as high active sites and facilitate the further surface modification, functionalization as well as the related applications.
The SAED pattern of the as-synthesized C3N4 nanowires is showed in Fig. 4. Five polycrystalline diffraction rings located at the d-spacing values of 2.39, 2.061, 1.473, 1.286 and 1.207 Å, which are corresponded to α-(201), β-(210), α-(311), β-(320) and β-(002) lattice planes of C3N4.30
The as-synthesized C3N4 nanowires are also studied by XRD (Fig. 5(a)), FTIR (Fig. 5(b)) and XPS (Fig. 5(c) and (d)). Five peaks at d-spacing of 2.389, 2.069, 1.465, 1.249 and 1.197 Å could be found in the typical XRD pattern of the samples, which were indexed as α-(201), β-(210), β-(301), α-(321) and β-(002) planes of the C3N4.30 Additionally, there is a broad peak at about 25.6° in the XRD pattern, indicating the existence of amorphous carbon in the as-synthesized C3N4 samples. The XRD results are in good agreement with that of FSEM, TEM, HRTEM and SAED characterizations. The FTIR spectrum reveals seven obvious absorption bands at about 3340, 1621, 1571, 1407, 1094, 936 and 802 cm−1. The broad absorption band near 3340 cm−1 can be ascribed to the stretching vibrations of O–H and/or N–H bonds. Three weak peaks at 1621, 1517 and 1407 cm−1 usually be assigned to the characteristic vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and sp3 C–C bonds, respectively. And the strong peak at 1094 cm−1 could be resulted from the stretching vibrations of C–N bond. The other two shoulder peaks at 936 and 802 cm−1 could be attributed to the out-of-plane flexural vibration of sp2 graphite structure and C–N–C group respectively. These peaks have frequently emerged in the FTIR spectrum of carbon nitride compounds and/or films.8,13,24,29,33,34 Fig. 5(c) and (d) show the typical C1s and N1s XPS spectra respectively. The C1s spectrum could be deconvoluted into three peaks at 285.7, 286.6 and 289.1 eV, attributed to sp2C–N, sp3C–N and C–O, respectively. The deconvoluted N1s peak consists of two kinds of nitride atom centered at 398.4, 399.8 and 401.3 eV, respectively corresponding to N–sp3C, N–sp2C and N–O.25,35–40 The XPS results demonstrate a good bonding status between C and N atoms in the as-synthesized C3N4 samples.
N and sp3 C–C bonds, respectively. And the strong peak at 1094 cm−1 could be resulted from the stretching vibrations of C–N bond. The other two shoulder peaks at 936 and 802 cm−1 could be attributed to the out-of-plane flexural vibration of sp2 graphite structure and C–N–C group respectively. These peaks have frequently emerged in the FTIR spectrum of carbon nitride compounds and/or films.8,13,24,29,33,34 Fig. 5(c) and (d) show the typical C1s and N1s XPS spectra respectively. The C1s spectrum could be deconvoluted into three peaks at 285.7, 286.6 and 289.1 eV, attributed to sp2C–N, sp3C–N and C–O, respectively. The deconvoluted N1s peak consists of two kinds of nitride atom centered at 398.4, 399.8 and 401.3 eV, respectively corresponding to N–sp3C, N–sp2C and N–O.25,35–40 The XPS results demonstrate a good bonding status between C and N atoms in the as-synthesized C3N4 samples.
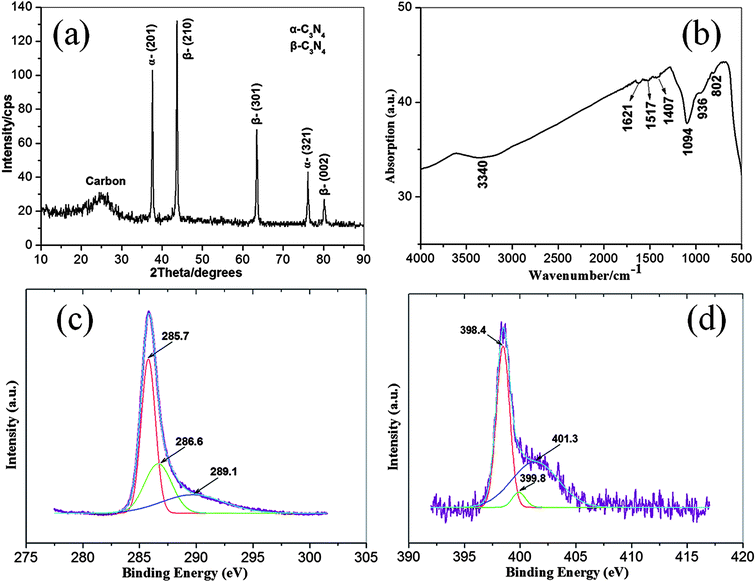 | ||
| Fig. 5 Typical XRD pattern (a), FTIR (b) and XPS (c and d) spectra of the as-synthesized C3N4 nanowires. | ||
3.2. Chemical reaction and growth mechanism
| [–CH2–CHCl–]n → C* + H2 + HCl | (1) |
| NH4Cl → N* + H2 + HCl | (2) |
| C* + N* + H2 + Fe2O3 → [C*–N*–Fe*] + H2O | (3) |
| [C*–N*–Fe*] → C3N4 + Fe | (4) |
| HCl + Fe → FeCl2 + H2 | (5) |
Firstly, PVC ([–CH2–CHCl–]n) began to decompose at about 130 °C, followed produced active vapor C*, H2 and HCl (eqn (1)) at high temperature.41–43 Meanwhile, NH4Cl was also dissociated into active vapor N* and H2 and HCl (eqn (2)).44–46 Then new generated chemically active C*, N* and H2 reacted with catalyst Fe2O3 and formed carbon-nitride-catalyst middle product [C*–N*–Fe*] liquid drop and H2O vapor (eqn (3), Fig. 6(a)).44,47 According to the VLS growth mechanism, with the help of catalyst Fe, C3N4 nanowires began to grow on the surface of [C*–N*–Fe*] when the C*and N* were supersaturated (eqn (4), Fig. 6(b)).48,49 This growth process will not be stopped only in the case that the active C*/N* was depleted or the catalyst Fe was inactivated (Fig. 6(c)). Finally, HCl reacted with Fe and produced FeCl2 and H2 (eqn (5)). Because of the different growth rates of the of the neighboring layers along the direction parallel to the crystal plane, the obtained C3N4 nanowires will possess interesting rough surface (Fig. 6(d)).
3.3 UV-visible (UV-vis) diffuse reflection spectrum and photoluminescence (PL) properties
Fig. 7(a) shows the UV-vis diffuse reflectance spectrum of the as-synthesized C3N4 nanowires. The band gap of the samples could be estimated to be about 4.38 eV (as shown in the inset of Fig. 7(a)). In fact, owing to the difference of the calculation method and accuracy, various theoretical band gap values had been reported in the previous studies. However, there also existed a common perspective that the α-C3N4 and β-C3N4 were both wide indirect band gap (3.0 eV ≤ Eg ≤ 7.0 eV) high-temperature semiconductors.3,22,39,50–53 The corresponding PL emission spectrum of the ethanol dispersion samples with an excitation wavelength of 230 nm was performed to study the optical property. As shown in Fig. 7(b), three emission peaks could be observed at 306 nm (4.05 eV), 330 nm (3.76 eV) and 340 nm (3.65 eV). The maximum emission peak centered at 340 nm suggested that the as-synthesized C3N4 nanowires were fluorescent but different from that of g-C3N4 (where the maximum emission peak was usually located at about 450 nm).54–59 In addition, the maximum emission peak of the samples was located in the ultraviolet wavelength region. This indicates that the as-synthesized C3N4 nanowires have potential applications as components of ultraviolet-light emitting nano-devices. The PL spectrum indicated that the samples could be used to fabricate ultraviolet luminescent devices and semiconductor lasers. | ||
| Fig. 7 Typical UV-vis diffuse reflectance spectrum (a) and the corresponding PL emission spectrum (b) of the as-synthesized C3N4 nanowires. | ||
4. Conclusions
In summary, high quality ultrafine α/β-C3N4 nanowires have been successfully fabricated through a novel hot-melt reduction synthetic route using low priced nontoxic raw materials. Relatively distinct and comprehensive characterization has been carried out to determine the intrinsic property of the α/β-C3N4 nanowires. The nanowires present good crystallinity with a length range of 1–4 μm and an average diameter of about 10 nm. Every nanowire possesses a high specific surface area and rough surface with abundant exposed atoms/prominences, predicting that the special surface structure will facilitate the further surface modification, functionalization and the related applications. The proposed catalytic reaction mechanism and the VLS growth model could properly interpret the formation process of the C3N4 nanowires. In addition, UV-vis diffuse reflectance and the corresponding PL spectra demonstrated a wide band gap (4.38 eV) and ultraviolet luminescent property of the α/β-C3N4 nanowires. The further research about the α/β-C3N4 nanowires will be worth expecting. We also hope our work could provide a reference for related research of the super-hard phase carbon nitride materials.Acknowledgements
This work was supported by the Program for New Century Excellent Talents in University (No. NCET-12-0655), Research Start-up Fund of Guilin University of Technology (No. 001210-002401003498), Guangxi Key Laboratory Open Fund in Universities of Clean Metallurgy and Comprehensive Utilization for Non-ferrous Metals Resources.References
- M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 32, 7988–7991 CrossRef CAS.
- A. R. Liu and M. L. Cohen, Science, 1989, 245, 841–842 CrossRef CAS PubMed.
- D. M. Teter and R. J. Hemley, Science, 1996, 271, 53–55 CrossRef CAS.
- L. W. Ruan, G. S. Xu, H. Y. Chen, Y. P. Yuan, X. Jiang, Y. X. Lu and Y. J. Zhu, J. Phys. Chem. Solids, 2014, 75, 1324–1333 CrossRef CAS.
- J. Z. Zhao and C. Z. Fan, Phys. B, 2008, 403, 1956–1959 CrossRef CAS.
- G. S. Manyali, R. Warmbier, A. Quandt and J. E. Lowther, Comput. Mater. Sci., 2013, 69, 299–303 CrossRef CAS.
- H. F. Dong, A. R. Oganov, Q. Zhu and G. R. Qian, Sci. Rep., 2015, 5, 9870 CrossRef CAS PubMed.
- Y. S. Wu and S. W. Wu, J. Alloys Compd., 2010, 489, 275–280 CrossRef CAS.
- T. Komatsu and T. Nakamura, J. Mater. Chem., 2001, 11, 474–478 RSC.
- E. G. Wang, J. Am. Ceram. Soc., 2002, 85, 105–108 CrossRef CAS.
- E. Broitman, N. Hellgren, O. Wanstrand, M. P. Johansson, T. Berlind, H. Sjostrom, J. E. Sundgren, M. Larsson and L. Hultman, Wear, 2001, 248, 55–64 CrossRef CAS.
- C. B. Cao, F. L. Huang, C. T. Cao, J. Li and H. Zhu, Chem. Mater., 2004, 16, 5213–5215 CrossRef CAS.
- J. Zhang, W. Liu, X. F. Li, B. Q. Zhan, Q. L. Cui and G. T. Zou, Mater. Res. Bull., 2009, 44, 294–297 CrossRef CAS.
- F. Z. Cui and D. J. Li, Surf. Coat. Technol., 2000, 131, 481–487 CrossRef CAS.
- Y. J. Guo and W. A. Goddard, Chem. Phys. Lett., 1995, 237, 72–76 CrossRef CAS.
- Z. H. Huang, B. Yang, C. S. Liu, L. P. Guo, X. J. Fan and D. J. Fu, Mater. Lett., 2007, 61, 3443–3445 CrossRef CAS.
- N. A. de Sanchez, C. Carrasco and P. Prieto, Phys. B, 2003, 337, 318–322 CrossRef CAS.
- J. X. Yang, F. Z. Cui, I. S. Lee, Y. P. Jiao, Q. S. Yin and Y. Zhang, Surf. Coat. Technol., 2008, 202, 5737–5741 CrossRef CAS.
- Z. Q. Li, J. Y. Zhou, J. Zhang, T. B. Chen and J. Yuan, J. Alloys Compd., 2002, 346, 230–234 CrossRef CAS.
- Y. S. Gu, Y. P. Zhang, Z. J. Duan, X. R. Chang, Z. Z. Tian, D. X. Shi, L. P. Ma, X. F. Zhang and L. Yuan, Mater. Sci. Eng., A, 1999, 271, 206–212 CrossRef.
- M. N. Uddin, O. A. Fouad, M. Yamazato and M. Nagano, Appl. Surf. Sci., 2005, 240, 120–130 CrossRef CAS.
- B. Molina and L. E. Sansores, Mod. Phys. Lett. B, 1999, 13, 193–201 CrossRef CAS.
- Q. A. Fu, C. B. Cao and H. S. Zhu, Chem. Phys. Lett., 1999, 314, 223–226 CrossRef CAS.
- Q. Lv, C. B. Cao, C. Li, J. T. Zhang, H. X. Zhu, X. Kong and X. F. Duan, J. Mater. Chem., 2003, 13, 1241–1243 RSC.
- X. F. Lu, L. G. Gai, D. F. Cui, Q. Wang, M. Zhao and X. T. Tao, Mater. Lett., 2007, 61, 4255–4258 CrossRef CAS.
- J. MartinGil, F. J. MartinGil, M. Sarikaya, M. X. Qian, M. JoseYacaman and A. Rubio, J. Appl. Phys., 1997, 81, 2555–2559 CrossRef CAS.
- Y. Kojima and H. Ohfuji, Diamond Relat. Mater., 2013, 39, 1–7 CrossRef CAS.
- L. W. Yin, Y. Bando, M. S. Li, Y. X. Liu and Y. X. Qi, Adv. Mater., 2003, 15, 1840–1844 CrossRef CAS.
- X. J. Bai, C. B. Cao, X. Y. Xu and Q. A. Yu, Solid State Commun., 2010, 150, 2148–2153 CrossRef CAS.
- S. Matsumoto, E. Q. Xie and F. Izumi, Diamond Relat. Mater., 1999, 8, 1175–1182 CrossRef CAS.
- M. J. Yacaman, J. M. Gil, F. J. M. Gil, M. Sarikaya and M. X. Qian, Mater. Chem. Phys., 1997, 47, 109–117 CrossRef CAS.
- L. L. Pang, J. Q. Bi, Y. J. Bai, Y. X. Qi, H. L. Zhu, C. G. Wang, J. W. Wu and C. W. Lu, Mater. Chem. Phys., 2008, 112, 1124–1128 CrossRef CAS.
- Z. X. Zhou, J. H. Wang, J. C. Yu, Y. F. Shen, Y. Li, A. R. Liu, S. Q. Liu and Y. J. Zhang, J. Am. Chem. Soc., 2015, 137, 2179–2182 CrossRef CAS PubMed.
- X. Lu, L. Gai, D. Cui, H. Jiang, Q. Wang, M. Zhao, X. Tao and M. Jiang, J. Cryst. Growth, 2007, 306, 400–405 CrossRef CAS.
- N. Hellgren, J. H. Guo, Y. Luo, C. Sathe, A. Agui, S. Kashtanov, J. Nordgren, H. Agren and J. E. Sundgren, Thin Solid Films, 2005, 471, 19–34 CrossRef CAS.
- F. R. Weber and H. Oechsner, Thin Solid Films, 1999, 355, 73–78 CrossRef.
- T. S. Wang, D. L. Yu, Y. J. Tian, F. R. Xiao, J. L. He, D. C. Li, W. K. Wang and L. Li, Chem. Phys. Lett., 2001, 334, 7–11 CrossRef CAS.
- D. Marton, K. J. Boyd, A. H. Albayati, S. S. Todorov and J. W. Rabalais, Phys. Rev. Lett., 1994, 73, 118–121 CrossRef CAS PubMed.
- V. N. Khabashesku, J. L. Zimmerman and J. L. Margrave, Chem. Mater., 2000, 12, 3264–3270 CrossRef CAS.
- M. Terrones, P. Redlich, N. Grobert, S. Trasobares, W. K. Hsu, H. Terrones, Y. Q. Zhu, J. P. Hare, C. L. Reeves, A. K. Cheetham, M. Ruhle, H. W. Kroto and D. R. M. Walton, Adv. Mater., 1999, 11, 655–658 CrossRef CAS.
- D. S. Wang, H. T. Sun, Q. Z. Luo, X. L. Yang and R. Yin, Appl. Catal., B, 2014, 156, 323–330 CrossRef.
- J. L. Wang, F. Long, W. M. Wang, S. Y. Mo, Z. G. Zou and Z. Y. Fu, Ceram. Int., 2016 DOI:10.1016/j.ceramint.2016.01.083.
- N. Miskolczi, L. Bartha and A. Angyal, Energy Fuels, 2009, 23, 2743–2749 CrossRef CAS.
- J. L. Wang, Y. L. Gu, Z. L. Li, W. M. Wang and Z. Y. Fu, Cryst. Growth Des., 2013, 13, 599–605 Search PubMed.
- J. Dai, L. Q. Xu, Z. Fang, D. P. Sheng, Q. F. Guo, Z. Y. Ren, K. Wang and Y. T. Qian, Chem. Phys. Lett., 2007, 440, 253–258 CrossRef CAS.
- J. L. Wang, X. Y. Pan and Y. L. Gu, Chem. J. Chin. Univ., 2010, 31, 239–242 CAS.
- J. L. Wang, Y. L. Gu, Z. L. Li, W. M. Wang and Z. Y. Fu, Diamond Relat. Mater., 2013, 31, 15–18 CrossRef CAS.
- J. L. Wang, L. P. Zhang, G. W. Zhao, Y. L. Gu, Z. H. Zhang, F. Zhang and W. M. Wang, J. Solid State Chem., 2011, 184, 2478–2484 CrossRef CAS.
- J. L. Wang, Y. L. Gu, Z. L. Li, X. W. Du, Z. X. Zhang, W. M. Wang, Y. C. Wang, H. Wang and Z. Y. Fu, CrystEngComm, 2014, 16, 2746–2753 RSC.
- Y. Xu and S. P. Gao, Int. J. Hydrogen Energy, 2012, 37, 11072–11080 CrossRef CAS.
- T. Y. Lu and J. C. Zheng, Chem. Phys. Lett., 2010, 501, 47–53 CrossRef.
- M. Mattesini, S. F. Matar and J. Etourneau, J. Mater. Chem., 2000, 10, 709–713 RSC.
- A. Y. Liu and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 10727–10734 CrossRef CAS.
- X. C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- K. Li, S. M. Gao, Q. Y. Wang, H. Xu, Z. Y. Wang, B. B. Huang, Y. Dai and J. Lu, ACS Appl. Mater. Interfaces, 2015, 7, 9023–9030 Search PubMed.
- J. Ma, B. L. Guo, X. T. Cao, Y. P. Lin, B. X. Yao, F. M. Li, W. Weng and L. Z. Huang, Talanta, 2015, 143, 205–211 CrossRef CAS PubMed.
- H. Li, C. B. Cao, H. W. Hao, H. L. Qiu, Y. J. Xu and H. S. Zhu, Diamond Relat. Mater., 2006, 15, 1593–1600 CrossRef.
- Y. C. Zhao, Z. Liu, W. G. Chu, L. Song, Z. X. Zhang, D. L. Yu, Y. J. Tian, S. S. Xie and L. F. Sun, Adv. Mater., 2008, 20, 1777–1781 CrossRef CAS.
- Q. Y. Lin, L. Li, S. J. Liang, M. H. Liu, J. H. Bi and L. Wu, Appl. Catal., B, 2015, 163, 135–142 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |


