Luminescence properties of single-phase color-tunable Li4SrCa(Si2O4N8/3):Eu2+ phosphor for white light-emitting diodes
Chenxia Lia,
Jian Daia,
Hua Yuc,
Degang Deng*b,
Jun Huangb,
Le Wanga,
Youjie Huab and
Shiqing Xu*b
aCollege of Optical and Electronic Technology, China Jiliang University, Hangzhou 310018, China
bCollege of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China. E-mail: dengdegang@cjlu.edu.cn; sxucjlu@163.com; Fax: +86-571-28889527; Tel: +86-571-86835781
cCollege of Materials and Environmental Engineering, Hangzhou Dianzi University, Hangzhou 310018, China
First published on 5th April 2016
Abstract
A novel single-phase color-tunable phosphor, Eu2+-activated Li4SrCa(Si2O4N8/3):Eu2+ (LSCSN:Eu2+), was synthesized via the conventional solid-state reaction method. The crystal structure of LSCSN has been determined by Rietveld refinement on X-ray powder diffraction data, and it was found that LSCSN crystallizes in an orthorhombic unit cell with the space group Pbcm(57). Under the excitation of UV light, the phosphor shows two main emission bands peaking at 430 nm and 578 nm, which have been confirmed to correspond to Eu2+ occupying the ten-coordination Sr sites and six-coordination Ca sites, respectively. Subsequently, the energy transfer between different Eu2+ emission centers was verified by the overlap of the emission and excitation spectra and the variation of fluorescence decay lifetimes. Meanwhile, when the excitation was changed to 375 nm light, it was found that the color hue can be tuned from blue to yellow, and even white if the Eu2+ ion concentration was increased to some extent. The thermal stability of the phosphor has been also investigated, which performs well with a high value of stability along with its integrated emission intensity remaining as 90.9% at 150 °C of that measured at room temperature. Furthermore, a warm white LED has been fabricated with a LSCSN:0.03Eu2+ phosphor and a 375 nm LED chip, and when the applied current is set to be 80 mA, the white LED was found to have a color rendering index (Ra) of 82.5 at the CIE coordinates of (0.3568, 0.3111) and correlated color temperature of 3318 K. The outstanding luminescence properties suggested that our prepared samples have potential application in w-LEDs.
1 Introduction
White light-emitting diodes (w-LEDs) have been attracting much attention because of their excellent merits such as long operational lifetime, high efficiency and low power consumption.1–3 Currently, the most popular approach to manufacturing w-LEDs is a combination of a blue InGaN LED chip with a yellow YAG:Ce3+ phosphor.4 Nevertheless, this approach can only obtain a cool white light due to the deficiency of sufficient red spectrum components in YAG:Ce3+, which restricts their applications in the areas of residential lighting and commercial lighting.5 The approach of combining UV (or n-UV) LED chips with tricolor (red, green, and blue) phosphors can produce warm white light with a higher color rendering index (CRI) and lower correlated color temperature (CCT), which has been regarded as a more effective route to meet the requirements of indoor lighting.6,7 However, according to existing experiments, the luminescence efficiency and emitting color stability do not perform well. The reasons lie in the emission re-absorption and thermal quenching behaviors of the three kinds of phosphors (red, green, and blue) involved, which are totally different, and also it is difficult to control the ratio and size of each phosphor.8–10 Recently, white light obtained by energy transfer from a sensitizer to an activator in a single component has been explored in the sense of convenient fabrication, high luminescence efficiency and good color reproduction. Based on this method, Eu2+/Mn2+, Eu2+/Ce3+ and Ce3+/Mn2+ are frequently employed as activators in the single-host with a two-color or three-color white light-emitting system.11–13 However, this method also has its drawbacks. For example, the existence of multiple activators leads to complexity of the experiment and decreases the stability of the phosphor at the same time.The exploration of approaches for achieving white light is still a hot topic in the field of luminescent materials.14 Considering the possibility of a single-host with full color emission by integrating a single-doped activator into different crystallographic sites, the single phosphor with a single activator model as a modest effective way to achieve white light is gaining lots of attention.15–18 This way can potentially solve the problems that appeared with the multiple activators model, and is deemed to have the ability to improve the luminescence efficiency. As a matter of fact, the existing explorations of a single-activator model have some disadvantages including low efficiency and less practicality, and the relevant experimental researches are rarely reported. Therefore, it is a crucial topic to develop a new single-phase solely-doped white light-emitting phosphor that is suitable for solid-state lighting.
It has been reported that the spectrum of Eu2+-doped Li4SrCa(SiO4)2 (hereafter referred to as LSCS) contains two emission bands which lie in the blue range and yellow range, respectively.19 However, according to the experimental result in the report, the intensity of the yellow peak is much weaker than that of the blue peak so it failed to generate satisfactory light. In our research, oxynitride Li4SrCa(Si2O4N8/3):Eu2+ (hereafter referred to as LSCSN:Eu2+) phosphors were prepared by nitrization of LSCS using Si3N4 as a nitrogen and silicon source. It is found that the yellow light emission and energy transfer between Eu2+ emission centers can be enhanced by this method, and a color-tunable light can be achieved. The crystal structure, Eu2+ site preference, and luminescence properties have been investigated in detail. This phosphor can be excited by n-UV light, and under such excitation the emitting light can be tuned from blue to white and then to yellow. Also, it can be used as a yellow phosphor under blue LED radiation. Lastly, a white LED was fabricated using the LSCSN:Eu2+ phosphor and a 375 nm UV chip, and the optical properties of the CRI, CCT and CIE of the fabricated LED have been investigated.
2 Experimental
2.1 Sample preparation
The LSCSN:xEu2+ (0 ≤ x ≤ 0.12) samples were synthesized by a conventional solid-state reaction method. The starting materials Li2CO3 (A.R.), SrCO3 (A.R.), CaCO3 (A.R.), Si3N4 (99.99%) and Eu2O3 (99.99%) were added in a stoichiometric ratio. After mixing and grinding, the powder mixture was put into BN crucibles and fired in a tube furnace. The powder mixture was pre-heated at 600 °C for 2 h and then sintered at 1000 °C for 4 h under a reducing atmosphere (5% H2/95% N2). The heating rate was 5 °C min−1. The final obtained phosphors were cooled down to room temperature and re-ground into powder for further measurements.2.2 White LED fabrication
White LEDs were fabricated by integrating a mixture of transparent silicon resin and a LSCSN:0.03Eu2+ phosphor on a 375 nm n-UV LED chip and roasting at 120 °C for 4 h afterward in an oven.2.3 Characterization
The phase identification of the LSCSN:Eu2+ samples was carried out by powder X-ray diffraction (XRD) (Bruker Axs D2 PHASER diffractometer, Cu Kα = 0.15406 nm) with 2θ ranging from 10° to 80°, operating at 30 kV and 10 mA. The surface morphology of the synthesized phosphor was observed with a field emission scanning electron microscope equipped with an energy-dispersive spectrometer (EDS) (FE-SEM, SU-8010, Hitachi, Japan). The excitation and emission spectra of the phosphors were measured on a PL3-211-P spectrometer (HORIBA JOBIN YVON, America) equipped with a 450 W xenon lamp as the excitation source. The fluorescence decay curve was performed employing this equipment with a pulsed spectral LED (370 nm) as excitation in multichannel scaling mode. The diffuse reflectance spectra were recorded with a UV-3600 UV-vis spectrometer (Shimadzu, Japan) using the white powder BaSO4 as a reference. The temperature dependence of photoluminescence was measured with excitation spectra and a thermal quenching analyzer for the phosphors (Everfine, China). The spectral, photometric and colorimetric quantities of the LEDs were measured by a HAAS-2000 light & radiation measuring instrument for packaged LEDs (Everfine, China).3 Results and discussion
3.1 Phase identification and crystal structure
The phase purities of the LSCSN:Eu2+ samples were verified using powder X-ray diffraction (XRD) and a Raman shift spectrum. Fig. 1(a) shows the experimental (black), refinement (red), and difference (green) XRD profiles for the Rietveld refinement of the LSCSN sample at room temperature. The LSCSN sample was refined by the computer software Topas program on the basis of XRD data, using the Li4SrCa(SiO4)2 structure as a starting model.20 The refined results show that the data are in good agreement with the starting model, which means that the crystal structure did not change obviously when O2− ions were substituted by N3− ions. The refinement data, fractional atomic coordinates, occupancies and beq parameters of this sample are shown in Table 1, and N3− is designed to substitute into the sites of O2−. The LSCSN sample obtained in this research crystallized in an orthorhombic unit cell with the space group Pbcm(57). The unit cell parameters (a = 4.98487 Å, b = 9.93483 Å, c = 14.0471 Å and V = 696.9828 Å3) differ from those of LSCS (a = 4.9832 Å, b = 9.9302 Å, c = 14.057 Å and V = 695.5633 Å3). The parameters a and b of LSCSN are larger than those of LSCS, but c is smaller. This is because there exists preferential occupation of N substituting O during the formation of the LSCSN solid solution.21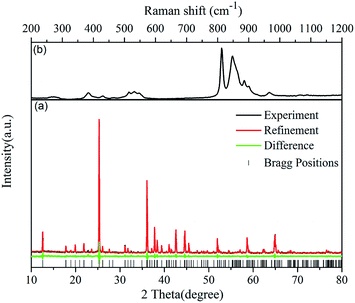 | ||
| Fig. 1 (a) Rietveld refinement XRD patterns of LSCSN (experiment: black line, refinement: red line and difference: green line), and (b) Raman shift spectrum. | ||
| Space group: Pbcm(57) (orthorhombic) | |
|---|---|
| Cell parameters | Reliability factors |
| Crystal density (g cm−3) = 9.005 | |
| Z = 4 | Rexp (%) = 4.79 |
| a = 4.98487 Å | Rwp (%) = 6.53 |
| b = 9.93483 Å | Rp (%) = 5.13 |
| c = 14.0471 Å | GOF = 1.36 |
| V = 696.9828 Å3 | |
| Atom | x | y | z | Occupancy | Beq |
|---|---|---|---|---|---|
| Sr1 | 0.05365 | 0.23221 | 0.74695 | 0.5 | 0.8302 |
| Ca1 | 0.50108 | 0.01602 | 0.50213 | 0.5 | 0.03975 |
| Si1 | 0.47215 | 0.97786 | 0.75003 | 0.5 | 2.617 |
| Si2 | 0.01987 | 0.23000 | 0.99466 | 0.5 | 0.5839 |
| Li1 | 0.95287 | 0.99715 | 0.86907 | 1 | −2.884 |
| Li2 | 0.51302 | 0.22184 | 0.62473 | 1 | 2.24 |
| (O/N)1 | 0.57198 | 0.30604 | 0.74128 | 1 | 2.113 |
| (O/N)2 | 0.80708 | 0.00385 | 0.75375 | 1 | 1.379 |
| (O/N)3 | 0.33230 | 0.04536 | 0.65633 | 1 | −0.3257 |
| (O/N)4 | 0.21414 | 0.33136 | 0.92530 | 1 | −0.121 |
| (O/N)5 | 0.81713 | 0.14147 | 0.55632 | 1 | 0.9946 |
The Raman shift spectrum (Fig. 1(b)) also shows that the phase of the synthesized phosphor is LSCSN. In the silicate compounds, the observed Raman peak at 268 cm−1 is mainly due to the Li–O framework and Li–O stretching and O–Li–O bending modes.22 The Raman peaks at 383, 400, 430 and 465 cm−1 are assigned to the Si–O–Si bending vibration mode. The broad band located around 500 cm−1 is mainly due to the O–Si–O rocking modes of tetrahedral SiO4, and the Si–O stretching bands are located around 800 cm−1.23 The 846–865 cm−1 band corresponds to the asymmetric in-plane Si–N stretching vibration mode.24 The Raman bands found between 885 and 1000 cm−1 are assigned to the Ca–Si–O and Sr–Si–O stretching vibration mode.25
The schematic structure of LSCSN is depicted in Fig. 2(a) according to the Rietveld refinement data; however, N and O atoms can’t be distinguished. In order to determine the location of N atoms in the LSCSN crystal structure, the cationic coordination environment was compared with and without N substitution. Fig. 2(b) and (c) show the coordination sphere of the Si, Li, Sr and Ca sites of LSCS and LSCSN, which are obtained from ICSD-79867 and Rietveld refinement, respectively. It can be seen that the two bond lengths of Sr–O5 vary from 3.003 to 3.129 and 3.045, respectively. Meanwhile, the two bond lengths of Ca–N4 vary from 2.414 to 2.593 and 2.299, respectively. Theoretically, the ionic bond length depends on the radius of the anion and cation, and the cation radius of N3− ions (1.46 Å) is larger than that of O2− ions (1.38 Å). When O2− ions are substituted by N3− ions, the corresponding bond length ought to become longer. Therefore, we could speculate that N3− would substitute O42− (connected to Ca) and O52− (connected to Sr) sites, as Fig. 2(c) shows. However, one of the Sr–N5 bonds was hardly changed and one of the Ca–N4 bonds got shortened obviously, which could be ascribed to the squeeze effect from longer Sr–N5 and Ca–N4 bonds during the formation of a substitutional solid solution.26 Moreover, compared with all of the Ca–O and Sr–O bond lengths, the bond length of Sr–O5 is the longest in the Sr coordination environment and the bond length of Ca–N4 is the longest in the Ca coordination environment. Therefore, they are the easiest ones to break and form a new bond. All these results point out that N3− ions occupy the O2− sites and are incorporated into the LSCSN lattice.
 | ||
| Fig. 2 (a) The schematic structure of LSCSN (black solid lines denote the unit cell). (b) and (c) The coordination environment of Sr and Ca sites in LSCS and LSCSN, respectively. | ||
The micromorphology of the crystalline LSCSN:0.04Eu2+ phosphor sample observed by SEM is shown in Fig. 3(a). It is observed that the particles have a smooth morphology and the diameters range from 10 to 25 μm. The energy-dispersive spectrum was employed to characterize the composition of the phosphor, as Fig. 3(b) shows. It confirms the formation of all samples by the synthesis method herein according to the presence of Sr, Ca, Si, N, O and Eu. However, it was hard to identify the light Li element by SEM-EDX measurement.
Fig. 4(a) shows the XRD patterns of the LSCSN:xEu2+ (0 ≤ x ≤ 0.12) samples and the standard data of JCPDS # 83-0763 (LSCS) as a reference. It is obvious that all the diffractions peaks of LSCSN:xEu2+ are well indexed to those of the standard card, suggesting that the obtained samples are of a pure phase. The doping Eu2+ ions have been incorporated into the host lattice without causing any significant effect on the crystal structure and nitridation. Fig. 4(b) shows the magnified XRD patterns in the region between 25° and 25.6°. It can be seen that the diffraction peak shifts to a higher angle first and then to a lower angle with the increase of Eu2+ concentration. As we know, the shift of diffraction peaks depends on the variation of lattice parameters. And in the host lattice of LSCSN, there is a ten-coordinated Sr2+ site (including eight oxygen atoms and two nitrogen atoms) and a six-coordinated Ca2+ site (including four oxygen atoms and two nitrogen atoms). The effective ionic radius of the six-coordinated Ca2+ is 1.00 Å and that of the ten-coordinated Sr2+ is 1.36 Å, while the effective ionic radii of the six- and ten-coordinated Eu2+ are 1.17 Å and 1.35 Å, respectively.27 The diffraction peaks shift to a higher angle for the substitutions of Eu2+ to Sr2+ and shift to a lower angle for the substitutions of Eu2+ to Ca2+. Therefore, according to the changed trend of the diffraction peaks, it is confirmed that Eu2+ has substituted into the Sr2+ and Ca2+ sites at the same time. The radius of Eu2+ is quite close to that of Sr2+, which means that the Sr2+ sites are more easily occupied by Eu2+ ions. This is the reason that the diffraction peak shifts to a higher angle first.
 | ||
| Fig. 4 (a) XRD patterns of LSCSN:xEu2+ (0 ≤ x ≤ 0.12) samples and the standard data of LSCS (JCPDS # 83-0763) as a reference. (b) Magnified XRD patterns in the region between 25° and 25.6°. | ||
3.2 Photoluminescence properties
Fig. 5 shows the excitation and emission spectra of the LSCSN:0.04Eu2+ phosphor. The excitation spectra monitored at 430 nm and 578 nm are both broad band with different peak shapes and intensity, which means that Eu2+ occupies two kinds of lattices sites (Sr and Ca sites). Under the excitation of ultraviolet (UV) light, the emission spectra show two emission bands with peak values at 430 nm and 578 nm, corresponding to the two Eu2+ ion emission centers. The shapes and peak values of the emission spectra are exactly the same under excitation of 325 nm and 375 nm, indicating that the introduction of N3− ions doesn’t change the number of cation sites substituted by Eu2+ ions. There are still only two Eu2+ emission centers occupying the Sr and Ca sites in the phosphor, named as Eu(Sr) and Eu(Ca) here for identification. This result is consistent with the previous structure discussion and XRD data analysis. According to the bond length shown in Fig. 2(c), the ten-coordination Sr position has a loose site (the average bond length was calculated to be 2.7646 Å) accommodating Eu2+ activators to correspond with a higher energy emission peak (Eu(Sr) site with a peak at 430 nm) and the six-coordination Ca position has a tight site (the average bond length was calculated to be 2.3693 Å) accommodating Eu2+ activators to correspond with a lower energy emission peak (Eu(Ca) site with a peak at 578 nm).20 Additionally, the distinct overlap between the excitation spectra monitored at 578 nm and the emission spectra excited at 325 nm proved the existence of energy transfer between the two kinds of Eu2+ emission centers.28 And, under excitation of 430 nm (the peak of the Eu(Sr) site), the phosphor shows strong yellow emission with a peak at 578 nm, which indicates that the Eu(Ca) site can absorb the emission of the Eu(Sr) site. This phenomenon further verified that the Eu(Sr) site can transfer energy to the Eu(Ca) site, and also indicated that the phosphor can be used as a yellow phosphor for blue LED chips. Upon excitation at a wavelength of 400 nm, the external quantum efficiencies of the optimized composition of LSCSN:0.04Eu2+ and commercial (Sr,Ba)2SiO4:Eu2+ (570 nm) phosphor were found to be 38.4% and 90% respectively. However, the quantum efficiency of LSCSN:0.04Eu2+ is 42.6% of that of the (Sr,Ba)2SiO4:Eu2+ (570 nm) phosphor. The lower quantum efficiencies of LSCSN:0.04Eu2+ could be further enhanced by process optimization.According to the report by Dorenbos,46 Eu2+ in a larger coordinating polyhedron would have a smaller crystal field splitting of the 5d levels. The polyhedron has a direct effect on the crystal field splitting, which can be expressed as an empirical formula:29
| εcfs = βpolyQRav−2 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βcubal(8)2+
βcubal(8)2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βcubo(12)2+ equals 1
βcubo(12)2+ equals 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89
0.89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.42, i.e., βpoly increases with the decreasing coordination number.30 The Rav values for Sr–(O/N) and Ca–(O/N) in LSCSN are 2.7646 Å and 2.3693 Å, respectively. Therefore, the crystal field splitting for the 5d levels of the Eu(Ca) site is larger than that for the Eu(Sr) site. Besides the crystal field splitting, the additional factor to influence the energy of the lowest 5d level is the centroid shift. The 5d level centroid shift can be estimated using the following equation:31,32
0.42, i.e., βpoly increases with the decreasing coordination number.30 The Rav values for Sr–(O/N) and Ca–(O/N) in LSCSN are 2.7646 Å and 2.3693 Å, respectively. Therefore, the crystal field splitting for the 5d levels of the Eu(Ca) site is larger than that for the Eu(Sr) site. Besides the crystal field splitting, the additional factor to influence the energy of the lowest 5d level is the centroid shift. The 5d level centroid shift can be estimated using the following equation:31,32
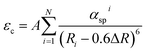 | (2) |
 | ||
| Fig. 6 Schematic diagram of energy transfer between the different Eu2+ emission centers (Sr2+ and Ca2+ sites) in the LSCSN:0.04Eu2+ phosphor. | ||
Fig. 7 shows the emission spectra of LSCS:0.04Eu2+ and LSCSN:0.04Eu2+ under 375 nm excitation. It is obvious that the emission spectra are slightly red-shifted (about 11 nm) due to the N3− ion substitution. As we known, a red-shift is caused by two elements: a centroid shift of the 5d orbital energy level and the effect of crystal field splitting. However, the cell volume becomes larger for N3− ions substituting O2− ions, which means that the crystal field splitting would not be the main reason for the red-shift. So, the red-shift results from the nephelauxetic effect, which can cause the change of the 5d band position.20 The difference of electronegativity between Eu2+ ions and N3− ions is smaller than that between Eu2+ ions and O2− ions, which means that the covalency of the N ligand is larger than that of the O ligand. The strong covalency would cause a larger centroid shift of the Eu2+ ions.33–35 Thus, the 5d energy levels of the Eu2+ ions are lowered, which led to the red-shift of the emission spectra. The emission intensity is enhanced with the N3− ions substituting for O2− ions, which is due to the local distortion of Eu2+ ions occurring when the larger N3− ions substitute the smaller O2− ions. The distorted host lattice around the Eu2+ ions would lead to an increase of the oscillator strength of the luminescence transition, i.e., increasing the probability of electron transition.36 And, the increase in the intensity of the yellow peak (Eu(Ca)) is greater than that of the blue peak (Eu(Sr)). This is because the incorporation of N3− ions leads to many more Ca sites substituted by Eu2+ ions and increases the energy transfer from the Eu(Sr) site to Eu(Ca) site at the same time.
Fig. 8 shows the diffuse reflection spectra of the undoped and Eu2+-doped LSCS and LSCSN samples. The diffuse reflection curves of the undoped LSCS and LSCSN are totally different, which also demonstrates that N3− ions are incorporated into the host lattice. Compared with that of LSCS:0.04Eu2+, the absorption band of LSCSN:0.04Eu2+ exhibits an obvious red-shift, which relates to the nephelauxetic effect and is in accordance with the emission intensity changing trend. The absorption enhancement is due to the introduction of N3− ions leading to the host lattice distortion around Eu2+ ions and increasing the probability of electron transition. At the same time, there is a probability that more Ca sites would be substituted by Eu2+ ions in the LSCSN host lattice, and Eu(Ca) sites can be effectively excited by n-UV and blue light.
It is well known that the Eu2+ ion concentration has significant influence on the energy transfer. Therefore, to investigate the effect of the Eu2+ ion concentration on the luminescence properties, a series of LSCSN:xEu2+ (0.01 ≤ x ≤ 0.12) samples were prepared. Fig. 9(a) shows the emission spectra of LSCSN:xEu2+ with different Eu2+ concentrations under excitation of 375 nm. The Eu2+ ion concentration quenching occurred at x = 0.07. The intensity of the peak at 430 nm gradually decreases, and the intensity of the peak at 578 nm gradually increases with the increasing Eu2+ ion concentration x, until x = 0.07. According to the percolation model, concentration quenching of the compound can occur by two mechanisms: (1) interactions between the Eu2+ ions, which result in energy re-absorption among neighboring Eu2+ ions in the rare earth sublattice; and (2) energy transfer from a percolating cluster of Eu2+ ions to killer centers.
 | ||
| Fig. 9 (a) The emission spectra and (b) relationships of log(I/xEu2+) versus log(xEu2+) in LSCSN:xEu2+ (0.01 ≤ x ≤ 0.12) under excitation of 375 nm. | ||
Hence we have calculated the critical distance between the Eu2+ ions for energy transfer using the relation given by Blasse:37
 | (3) |
The non-radiative energy transfer between Eu2+ ions is caused by electric multipole–multipole interactions, which depend on distance according to Dexter’s theory.38 The strength of the multipole–multipole interactions can be determined from the change in the emission intensity if the energy transfer occurs between the same types of activators. The emission intensity (I) per activator ion is expressed by the formula:39,40
| I/x = k[1 + β(x)θ/3]−1 | (4) |
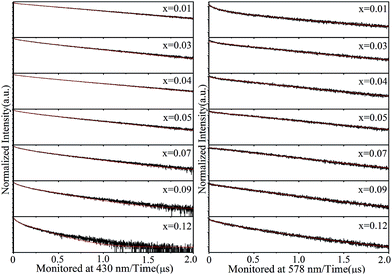
The energy transfer can also be demonstrated from the decay properties. Fig. 10 illustrates the fluorescence decay curves of the LSCSN:xEu2+ (0.01 ≤ x ≤ 0.12) samples at room temperature under excitation of 370 nm. The decay curves can be best-fitted by the following double exponential equation:41
I = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2)
| (5) |
| 〈τ*〉 = (A1τ12 + A2τ22)/(A1τ1 + A2τ2) | (6) |
The calculated decay times are shown in Fig. 11. The decay times monitored at 430 nm decrease with the increasing Eu2+ ion concentration, and the decay times monitored at 578 nm increase with the increasing Eu2+ ion concentration and reach a maximum value when x = 0.07, then decrease. Those results confirm that energy transfer from Eu2+ ions located in the Sr sites to Eu2+ ions located in the Ca sites has taken place,42 and the decay times decrease when x exceeds 0.07, due to the concentration quenching.
 | ||
| Fig. 11 The calculated decay times of the LSCSN:xEu2+ (0.01 ≤ x ≤ 0.12) phosphor monitored at 430 nm and 578 nm. | ||
Fig. 12 shows the CIE chromaticity coordinates of the LSCSN:xEu2+ (0.01 ≤ x ≤ 0.12) phosphors under excitation of 375 nm. With the increase of the concentration of Eu2+ ions, the color hue tunes from blue (point 1) to yellow (point 7) and the corresponding chromaticity coordinates ranging from (0.2770, 0.2028) to (0.4792, 0.4495) are summarized in the inset table. When the concentration of Eu2+ ions was x = 0.03 and 0.04, white light was obtained with CIE of (0.3387, 0.3153) and (0.3537, 0.3385), respectively. Therefore, the Eu2+-doped LSCSN phosphor can be used as a color-tunable and white light-emitting phosphor.
 | ||
| Fig. 12 The CIE chromaticity coordinates of the LSCSN:xEu2+ (0.01 ≤ x ≤ 0.12) phosphors under excitation of 375 nm. | ||
3.3 Thermal stability
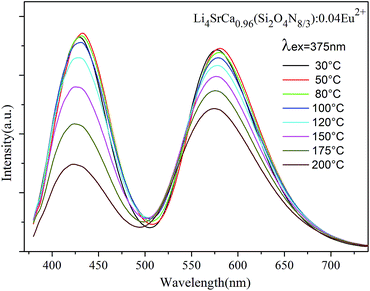
It is well known that the thermal stability of LED phosphors is a critical parameter, which is used to balance phosphor conversion of white LED performance. So the temperature dependence of the luminescence was investigated. Fig. 13 presents the emission spectra (excited at 375 nm) of LSCSN:0.04Eu2+ at different temperatures and Fig. 14 shows the intensity of two emission peaks and the integrated intensity with the temperature range 30–200 °C. The emission intensities of the two peaks show almost no decrease when the temperature is 80 °C, which is because the high temperature causes the expansion of the unit and enhances the symmetry of the host lattice.43 When the temperature is up to 150 °C, the normalized emission intensity of the peaks at 430 nm and 578 nm decreases to 86.8% and 76.8% of the initial value, respectively. And the integrated intensity decreases to 90.9% of the initial value with the temperature up to 150 °C. Those results obviously indicate that the LSCSN:Eu2+ phosphor has very good temperature stability and color stability. The difference decay of the two peaks is because of the existence of energy transfer from a higher energy center to a lower energy center, which leads to the lower energy emission decrease slowly. Compared with the commercial (Sr,Ba)SiO4:Eu2+ (570 nm) phosphor, the phosphor in our study shows better thermal stability.44 | ||
| Fig. 14 The normalized emission intensity of the peaks and integrated intensity as a function of temperature. | ||
3.4 Optical properties of the LED lamp
To demonstrate the potential application of LSCSN:Eu2+, a white LED consisting of a LSCSN:0.03Eu2+ phosphor and a 375 nm LED chip was fabricated. Fig. 15 shows the electroluminescence (EL) spectrum under a different forward bias current, and the inset (a) shows pictures of the white LED lamp and (b) shows the LED in operation. It is obvious that the emission intensity is enhanced with the applied current increased from 20 mA to 180 mA. The CIE coordinates, CCT and Ra are shown in Table 2 under the applied currents. The CCT was decreased with the increasing current, indicating that the degree of increase of the yellow emission is larger than that of the blue emission. When the applied current was 80 mA, the white LED had CIE color coordinates of (0.3568, 0.3111) and achieved a Ra of 82.5 around the correlated color temperature of 3318 K. Compared with the YAG:Ce3+ phosphor pumped by a blue InGaN chip (CCT = 7756 K, Ra = 75),45 the white LED in this study shows a lower CCT value and a higher Ra value. The result demonstrates that LSCSN:0.03Eu2+ can be used as a single-phase warm white light-emitting phosphor.| Forward bias current | CIE coordinates | CCT (K) | Ra | |
|---|---|---|---|---|
| x | y | |||
| 20 mA | 0.3511 | 0.3006 | 3387 | 83.9 |
| 50 mA | 0.3540 | 0.3039 | 3348 | 82.8 |
| 80 mA | 0.3568 | 0.3111 | 3318 | 82.5 |
| 120 mA | 0.3565 | 0.3129 | 3304 | 81.9 |
| 180 mA | 0.3662 | 0.3223 | 3216 | 81.0 |
4 Conclusions
In summary, a novel single-phase color-tunable phosphor, Eu2+-activated LSCSN:Eu2+, was synthesized via the conventional solid-state reaction method. The crystal structure and luminescence properties were discussed. LSCSN crystallizes in an orthorhombic unit cell with the space group Pbcm(57) and unit cell parameters a = 4.98487 Å, b = 9.93483 Å, c = 14.0471 Å and V = 696.9828 Å3. Under excitation of UV light, the phosphor exhibited two main emission bands peaking at 430 nm and 578 nm, which correspond to Eu2+ occupying the ten-coordination Sr sites and six-coordination Ca sites, respectively. The phosphor also can be effectively excited by blue light and can be used as a yellow phosphor for blue LED chips. The energy transfer between different Eu2+ ion emission centers was confirmed by the overlap of the emission and excitation spectra and the variation of the fluorescence decay lifetimes. Under 375 nm excitation, the color hue can be tuned from blue to white then to yellow with the increase of the Eu2+ ion concentration. The phosphor showed good thermal stability and the integrated emission intensity remained at 90.9% at 150 °C of that measured at room temperature. Furthermore, a warm white LED was fabricated with the LSCSN:0.03Eu2+ phosphor and a 375 nm LED chip. When the applied current was 80 mA, the white LED had a CIE of (0.3568, 0.3111) and achieved a Ra of 82.5 around the correlated color temperature of 3318 K.Acknowledgements
This research is supported by the Project of the National Nature Science Foundation of China under Grant No. 51272243, 61405185, 61370049, 51402077 and 61575182, Zhejiang Provincial Natural Science Foundation of China under Grant No. LR14A040002, LQ13E020003 and Y16F050012.References
- M. R. Krames, O. B. Shchekin, R. Mueller-Mach, G. O. Mueller, L. Zhou, G. Harbers and M. G. Craford, J. Disp. Technol., 2007, 3, 160–175 CrossRef CAS.
- N. Yukio, N. Junya, S. Takahiko, Y. Takao, N. Hiroki, S. Masahiko and M. Takashi, Phys. Status Solidi, 2007, 204, 2087–2093 CrossRef.
- E. F. Schubert and J. Kim, Science, 2005, 308, 1274–1278 CrossRef CAS PubMed.
- S. Lee and S. Y. Seo, J. Electrochem. Soc., 2002, 149, J85–J88 CrossRef CAS.
- N. Komuro, M. Mikami, Y. Shimomura, E. G. Bithell and A. K. Cheetham, J. Mater. Chem. C, 2014, 2, 6084–6089 RSC.
- N. C. George, K. A. Denault and R. Seshadri, Annu. Rev. Mater. Res., 2013, 43, 481–501 CrossRef CAS.
- H. J. Song, D. K. Yim, H. S. Roh, I. S. Cho, S. J. Kim, Y. H. Jin, H. W. Shim, D. W. Kim and K. S. Hong, J. Mater. Chem. C, 2013, 1, 500–505 RSC.
- C. H. Huang, Y. C. Chiu, Y. T. Yeh, T. S. Chan and T. M. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 6661–6668 CAS.
- D. Deng, H. Yu, Y. Li, Y. Hua, G. Jia, S. Zhao, H. Wang, L. Huang, Y. Li, C. Li and S. Xu, J. Mater. Chem. C, 2013, 1, 3194–3199 RSC.
- X. Li, J. D. Budai, F. Liu, J. Y. Howe, J. Zhang, X. J. Wang, Z. Gu, C. Sun, R. S. Meltzer and Z. Pan, Light: Sci. Appl., 2013, 2, e50 CrossRef.
- C. H. Wang, W. R. Liu and T. M. Chen, J. Phys. Chem. C, 2010, 114, 18698–18701 Search PubMed.
- G. Li, D. Geng, M. Shang, C. Peng, Z. Cheng and J. Lin, J. Mater. Chem., 2011, 21, 13334–13344 RSC.
- L. Chen, A. Luo, Y. Zhang, F. Liu, Y. Jiang, Q. Xu, X. Chen, Q. Hu, S. F. Chen, K. J. Chen and H. C. Kuo, ACS Comb. Sci., 2012, 14, 636–644 CrossRef CAS PubMed.
- Y. Wang, H. Li, R. Zhang, M. Zhan and J. Zhang, RSC Adv., 2015, 5, 2689–2693 RSC.
- H. He, R. Fu, X. Song, D. Wang and J. Chen, J. Lumin., 2008, 128, 489–493 CrossRef CAS.
- J. Y. Han, W. B. Im, D. Kim, S. H. Cheong, G. Y. Lee and D. Y. Jeon, J. Mater. Chem., 2012, 22, 5374 RSC.
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372–1386 RSC.
- T.-G. Kim, T. Kim, J. Kim, S.-J. Kim and S.-J. Im, J. Phys. Chem. C, 2014, 118, 12428–12435 CAS.
- J. Zhang, W. Zhang, Y. He, W. Zhou, L. Yu, S. Lian, Z. Li and M. Gong, Ceram. Int., 2014, 40, 9831–9834 CrossRef CAS.
- Z. Zhao, Z. Yang, Y. Shi, C. Wang, B. Liu, G. Zhu and Y. Wang, J. Mater. Chem. C, 2013, 1, 1407–1472 RSC.
- T. Wang, J. Yang, Y. Mo, L. Bian, Z. Song and Q. L. Liu, J. Lumin., 2013, 137, 173–179 CrossRef CAS.
- Y. Repelin, E. Husson, F. Bennani and C. Proust, J. Phys. Chem. Solids, 1999, 60, 819–825 CrossRef CAS.
- L. Galeener, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 19, 4292–4297 CrossRef.
- G. J. Wan, N. Huang, P. Yang, R. K. Y. Fu, J. P. Y. Ho, X. Xie, H. F. Zhou and P. K. Chu, Mater. Sci. Eng., C, 2007, 27, 928–932 CrossRef CAS.
- F. L. Galeener, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 19, 4292–4297 CrossRef CAS.
- X. J. Li, H. P. Ma, D. Deng and S. Xu, CrystEngComm, 2015, 17, 9123–9134 RSC.
- R. D. Shannon, Acta Crystallogr., Sect. A: Found. Adv., 1976, 32, 751–767 CrossRef.
- P. Li, Z. Wang, Z. Yang and Q. Guo, J. Mater. Chem. C, 2014, 2, 7823–7829 RSC.
- L. Uitert, J. Lumin., 1984, 29, 1–9 CrossRef.
- J. Zhang, W. Zhang, Z. Qiu, W. Zhou, L. Yu, Z. Li and S. Lian, J. Alloys Compd., 2015, 646, 315–320 CrossRef CAS.
- P. Dorenbos, J. Phys.: Condens. Matter, 2003, 15, 4797–4807 CrossRef CAS.
- J. Huang, J. Dai, D. Deng, H. Yu, Y. Li, Y. Hua, S. Zhao, C. Li and S. Xu, RSC Adv., 2015, 5, 85682–85690 RSC.
- W. Tang and H. Xue, RSC Adv., 2014, 4, 62230–62236 RSC.
- Z. Wang, Z. Xia, M. S. Molokeev, V. V. Atuchin and Q. L. Liu, Dalton Trans., 2014, 43, 16800–16804 RSC.
- T. Wang, Z. Xia, Q. Xiang, S. Qin and Q. Liu, J. Lumin., 2015, 166, 106–110 CrossRef CAS.
- K. E. Waldrip, J. S. Lewis, Q. Zhai, M. Puga-Lambers and M. R. Davidson, J. Appl. Phys., 2001, 89, 1664–1667 CrossRef CAS.
- G. Blasse, Philips Res. Rep., 1969, 24, 131 CAS.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836 CrossRef CAS.
- L. G. Van Uitert, J. Electrochem. Soc., 1967, 114, 1048 CrossRef CAS.
- L. Ozawa and P. M. Jaffe, J. Electrochem. Soc., 1971, 118, 1678 CrossRef CAS.
- H. Yu, D. Deng, L. Chen, D. Chen, J. Zhong, H. Zhao and S. Xu, Ceram. Int., 2015, 41, 3800–3805 CrossRef CAS.
- W. Sun, Y. Jia, R. Pang, H. Li, T. Ma, D. Li, J. Fu, S. Zhang, L. Jiang and C. Li, ACS Appl. Mater. Interfaces, 2015, 7, 25219–25226 CAS.
- Z. Xia, X. Wang, Y. Wang, L. Liao and X. Jing, Inorg. Chem., 2011, 50, 10134–10142 CrossRef CAS PubMed.
- J. S. Kim, Y. H. Park, S. M. Kim, J. C. Choi and H. L. Park, Solid State Commun., 2005, 133, 445–448 CrossRef CAS.
- Y. Hua, H. Ma, D. Deng, S. Zhao, L. Huang, H. Wang and S. Xu, J. Lumin., 2014, 148, 39–43 CrossRef CAS.
- P. Dorenbos, Crystal field splitting of lanthanide 4fn–15d-levels in inorganic compounds, J. Alloys Compd., 2002, 341, 156–159 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |