A fluorescent, self-healing and pH sensitive hydrogel rapidly fabricated from HPAMAM and oxidized alginate with injectability†
Wen Yanga,
Xiaotian Wua,
Fangbing Liua,
Yan Doua,
Zhenhu Hub and
Wentao Hao*a
aDepartment of Polymer Materials and Engineering, School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei, P. R. China 230009. E-mail: wentao_hao@hfut.edu.cn
bDepartment of Municipal Engineering, School of Civil Engineering, Hefei University of Technology, Hefei, P. R. China 230009
First published on 31st March 2016
Abstract
Multifunctional hydrogels are very interesting, although they might need complex procedures to achieve this goal. Here we report an easily fabricated injectable hydrogel, which is fluorescent, self-healing, pH-sensitive and biodegradable, from hyperbranched poly(amido amine) (HPAMAM) and oxidized alginate (OALG). The HPAMAMs are fluorescent, easy to flow, amine functionalized and biocompatible. OALGs are oxidized products of ALG, a natural polymer, with abundant aldehyde groups. When the two components were injected together, they gelled within 1 minute. The electrostatic force, hydrogen bonds and acylhydrazone bonds are the bases of the HPAMAM/OALG hydrogel. All of them are dynamic in nature. Therefore, this kind of hydrogels is naturally able to heal themselves, sensitive to the variation of pH, and biodegrade after finishing their task. The HPAMAM/OALG hydrogels are of suitable strength and porous structure, which make them good candidates in biomedical applications, from tissue engineering to sustained drug delivery.
1. Introduction
Injectable hydrogels have attracted tremendous attention from researchers because of their remarkable properties including accurate-targeting implantation and minimized injury, in addition to their great potentials in tissue engineering and controlled drug release.1–3 During the past several years, a lot of injectable hydrogels with novel functionality have emerged, for instance, fluorescent,4–7 self-healing,8–11 and stimuli-responsive injectable hydrogels.12,13 The fluorescent hydrogels have prospective applications in tissue engineering as cell differentiation and proliferation can be visualized in situ.14 In addition, they can be applied in fluorescence-guided monitoring and surgical resection.15 The self-healing hydrogels are able to spontaneously maintain the shape and structural integrity of the implants.9,16 The stimuli-responsive hydrogels may release drugs very quickly in responding to specific environment stimulus.12,13 However, the multifunctional hydrogels integrating fluorescence, self-healing ability and stimuli-responsibility were still rare. Moreover, it might need complex procedures to achieve that goal.17Hyperbranched poly(amido amine)s (HPAMAMs) are known for their ability to emit fluorescence under UV irradiation.18,19 Like their dentrimeric analogous, the HPAMAMs are of compact structure, easy to flow, which is the key to the inject ability of the hydrogels. Another structural feature of HPAMAMs is that there are abundant amine groups on periphery of these spherical molecules. They can react with the aldehyde groups on the oxidized products of alginate (OALG), a natural polymer, to form acylhydrazone bonds. The acylhydrazone bonds or Schiff bases have been approved to be dynamic covalent bonds, which can reversibly break and recombine together.20,21 Furthermore, the Schiff bases are sensitive to the pH values.22,23 In very low pH circumstance, they will break down irreversibly. Therefore, it can be reasonably speculated that injectable hydrogels formed by HPAMAM and OALG might possess good fluorescent property, self-healing ability, pH-sensitivity and biodegradation ability.
In this work, HPAMAM were synthesized through Michael addition polymerization from N,N′-methylenebisacrylamide (MBA) and 1-(2-aminoethyl)piperazine (AEPZ). When the aqueous solutions of HPAMAM and OALG were injected together, hydrogels can be formed simultaneously. As expected, this kind of injectable hydrogel is able to emit fluorescence under UV irradiation. They can also self-heal at room temperature. Moreover, it was shown that the HPAMAM/OALG hydrogel was of potential to release drugs. Due to their sensitivity to acidic environment, they are very suitable for targeted therapy of tumour. These hydrogels were of suitable strengths that they might function as tissue engineering scaffold. Lastly, the hydrogels could be degraded gradually. In another word, they can be metabolized naturally by the living bodies without being taken out surgically right after the mission accomplished.
2. Experimental
2.1 Materials
N,N′-Methylenebisacrylamide (MBA) and 1-(2-aminoethyl)-piperazine (AEPZ) were purchased from Aladdin Reagents. Sodium alginate, sodium periodate, methanol, ethanol, ethylene glycol, phenolphthalein, concentrated HCl solution, triethylamine and sodium hydroxide were obtained from Sinopharm Reagents and used as received without further purification.2.2 Preparation of HPAMAM/OALG hydrogel
HPAMAM was prepared by Michael addition in a procedure similar to the previous reports.24–26 Typically, MBA (10.175 g, 0.066 mol) and AEPZ (8.527 g, 0.066 mol) were added into a 120 mL methanol/water mixture (7/3, v/v) under gentle stirring. The polymerization was carried out at 50 °C for 5 days. Then the reactant was precipitated in cold acetone twice. The solids were collected by filtration. Lastly, the products were dried in a vacuum oven for 12 hours. The molecular weight was about 8.2 × 103 g mol−1.OALG was prepared according to previously reported methods.27 Sodium alginate was oxidized with sodium periodate at room temperature. 10 g sodium alginate was dissolved in 500 mL deionized water, and then 8.56 g sodium periodate was dissolved in 100 mL deionized water. The two solutions were mixed together and stirred for 6 hours. The reaction was halted by addition of ethylene glycol. After precipitation with large amount of ethanol, the oxidized product of sodium alginate (OALG) was collected and dialyzed. Finally, the OALG was freeze-dried. The oxidation degree was about 80%.
OALG (0.05 g mL−1) and HPAMAM (0.15 g mL−1) aqueous solutions were mixed in equivalent volume. Within 1 minute, the mixed solutions gelled. The obtained hydrogels were named as Gel-i. When using the 0.10, 0.15 and 0.20 g mL−1 OALG solutions and the HPAMAM solution of the same concentration, the obtained hydrogels were named as Gel-ii, Gel-iii and Gel-iv, respectively.
2.3 Characterizations
Gelation time of the hydrogels was characterized by a viscometer (model LVDV-II+P, Brookfield) operating at 30 rad s−1. FTIR analysis of the HPAMAM/OALG hydrogel was performed on a Nicolet iS10, Thermoscientific. Morphology of the freeze-dried hydrogel was observed on a SEM model JSM-6700F, JEOL. Fluorescence spectra of hydrogels were recorded on a spectrometer model F-2700, Hitachi.Rheological behaviour of hydrogels was measured using an AR 1000 rheometer from TA Instruments at room temperature. A 4 cm diameter parallel plate was used within the viscoelastic region in experiments. The storage moduli G′ and loss moduli G′′ were measured as a function of the frequency (rad s−1).
Self-healing ability of the HPAMAM/OALG hydrogel was tested at room temperature. No stimulus, like pH adjustment or heat was applied. Two pieces of hydrogel were prepared and one of them was dyed into red by rhodamine B. After they were cut into halves, the halves were crossly recombined together. The pH sensitivity of the hydrogel was tested by addition of concentrated HCl solution (1 M) and triethylamine sequentially.
Dynamic swelling behaviours of hydrogels were investigated by their swelling ratios in PBS buffers (pH 7.4). Briefly, hydrogels were weighed after being freeze-dried (M0), followed by swelling in buffers. At predetermined time points, the swollen samples were taken out. After quickly removing the excess water, the hydrogels were weighed (Mt). Then, the samples were returned to the containers with refilling fresh buffer to original volume. The swelling ratio of hydrogels was calculated by (Mt − M0)/M0 × 100%. All experiments were repeated 3 times.
Degradation of HPAMAM/OALG hydrogels was performed in PBS buffer (pH = 5). Before being immersed into the buffer, the hydrogels were freeze-dried and weighed (M0). At predetermined time points, the gels were taken out and freeze dried, then weighted again (Mn). The degradation ratio was calculated by Mn/M0 × 100%. All experiments were repeated 3 times.
Rhodamine B was used as the model drug to evaluate the drug release ability of the hydrogels. Rhodamine B (0.025 mL, 0.001 g mL−1) was added into HPAMAM solution (0.5 mL, 0.15 g mL−1), and stirred at room temperature for 1 day. After that, the solution was mixed with equivalent volume of OALG solution (0.05 g mL−1). The obtained hydrogels were incubated in 6 mL PBS buffers with various pH values (2, 5, and 7.4) at 37 °C. At each predetermined time points, 4 mL of sample buffer were taken out for UV-vis analysis at 555 nm and then the same volume of fresh buffer was put back into the incubator. All experiments were repeated 3 times.
3. Results and discussion
3.1 Formation of HPAMAM/OALG injectable hydrogel
Hydrogels from HPAMAM and OALG can be fabricated very quickly after mixing the aqueous solutions of the two components (Fig. 1a–c). Within 1 minute, the gels were formed. The mixture solution of HPAMAM and OALG gelled faster than that in the previous reports.28,29 The fast gelation process was evidenced by the viscosity measurements (Fig. S1†). Thirty seconds later after the HPAMAM (0.15 g mL−1) and OALG (0.1 g mL−1) solutions were mixed together, the viscosity increased drastically, indicating the formation of hydrogel networks. As shown in Fig. 1d–f, gel thread could be simultaneously fabricated when injecting the OALG solution into HPAMAM solution. The very fast formation of HPAMAM/OALG gel was very useful. It suggests that the gel can be implanted into the body efficiently. It was supposed that there were several different interactions among the HPAMAM and OALG. Firstly, there were electrostatic interactions among the positively charged HPAMAM and negatively charged OALG. Secondly, there were hydrogen bonds among the two components. Thirdly, the HPAMAM and OALG could establish chemical crosslinks via reaction between –NH2 groups on HPAMAM and –CHO groups on OALG (Fig. S2 and S3†).The HPAMAM used was synthesized from AEPZ and MBA via Michael addition polymerization. As a result, there are abundant amine (–NH2) groups on the spherical surface of HPAMAM molecules. The OALGs, which were fabricated from oxidation of alginates, are of many aldehyde groups (–CHO). The –NH2 groups can form hydrogen bonds with the –OH groups on the OALG. Moreover, they are highly reactive to the –CHO groups, leading to the formation of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds between these two polymers, which was evidenced by the FTIR analysis,30,31 shown in Fig. S3.† It was thought that the HPAMAM physically gelled with OALG through electrostatic force and hydrogen bonds at first, and then the gel was strengthened by chemical links. A schematic diagram was shown in Fig. 2. These interactions between HPAMAM and OALG were essential not only to the fast gelation speed, but also to the final properties of the hydrogels, like self-healing ability, pH-sensitivity, and degradation ability.
N– bonds between these two polymers, which was evidenced by the FTIR analysis,30,31 shown in Fig. S3.† It was thought that the HPAMAM physically gelled with OALG through electrostatic force and hydrogen bonds at first, and then the gel was strengthened by chemical links. A schematic diagram was shown in Fig. 2. These interactions between HPAMAM and OALG were essential not only to the fast gelation speed, but also to the final properties of the hydrogels, like self-healing ability, pH-sensitivity, and degradation ability.
 | ||
| Fig. 2 Schematic diagram of the electrostatic interactions (physically) and Schiff-bases (chemically) between HPAMAM and OALG. The H-bonds were not illustrated. | ||
3.2 Fluorescent property of HPAMAM/OALG hydrogels
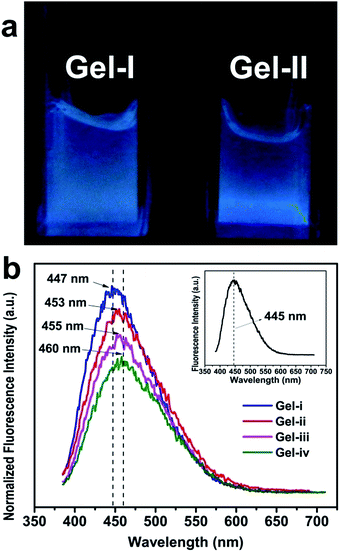
As indicated in Fig. 3a, the HPAMAM/OALG hydrogels emitted bright blue light under UV irradiation (365 nm). In Fig. 3b, it was shown that the emission peaks of HPAMAM/OALG hydrogels were almost in the same positions as that of the HPAMAM.It suggested that the fluorescent property of the hydrogels was inherited from HPAMAM molecules. However, a slight shift towards higher wavelengths of emission peaks was found (Fig. 3b). That might be due to the molecular motion of PAMAM molecules being restricted.32 When the HPAMAM and OALG solutions were mixed together, the mixed solution quickly turned into a gel. In the gel, the molecular motion of HPAMAM was suppressed because of the formation of electrostatic interactions, hydrogen bonds and acylhydrazone bonds between the polymers. Furthermore, the HPAMAM/OALG hydrogels showed multiple emissions depending upon the excitation wavelengths (Fig. S4a†). Such a spectrum was similar to that of the HPAMAM solutions (Fig. S4b†). Generally, it was thought that there were multiple states of excitation.33
3.3 Self-healing ability of HPAMAM/OALG hydrogels
Dynamic rheological measurements can give some useful information of the self-healing ability of hydrogels.34,35 As shown in Fig. 4, the storage moduli (G′) of the HPAMAM/OALG hydrogels decreased slightly in very low frequency region, whereas the loss moduli (G′′) increased. A cross point could be expected although it was out of the range of the instrument. The cross point of G′ and G′′ was thought to be a symbol of self-healing ability of hydrogels, which suggests the collapse of dynamic networks.34,35 As indicated in Fig. 5, the two pieces of HPAMAM/OALG hydrogel could self-heal at room temperature. It took only several minutes for the severed hydrogels to recombine together. Two hours later, the recombined pieces could withstand their own weight. However, the stress–strain curves of the self-healed samples were not obtained at the present time. The hydrogels are weak. It is hard to tightly grip the gel samples on clamps of the testing machine. They always broke. However, the gel samples might slip out when loosened the clamps. | ||
| Fig. 4 Storage moduli (G′) and loss moduli (G′′) vs. angular frequency (ω) of the HPAMAM/OALG hydrogels. | ||
During the self-healing experiments of the HPAMAM/OALG hydrogel samples (Fig. 5c and d), there was no stimulus, like pH adjustment or heat being used. It is interesting that the severed hydrogel samples recombined together without changing the pH value. Usually, the acylhydrazone bonds can dynamically break and recombine at weak acidic environments.34 However, the pH of these hydrogels was about 8–8.5, which was determined by the pH test paper. That is, the fast self-healing of the HPAMAM/OALG hydrogel might not the result of dynamic exchange of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds. To test that hypothesis, we changed the pH value of the HPAMAM solution to more than 9.0 and mixed it with the OALG solution to fabricate an alkaline hydrogel. It was found that the hydrogel sample could still self-heal (Fig. S5†). It is supposed that the electrostatic interactions and hydrogen bonds between the HPAMAM and OALG molecules, in addition to the hydrogen bonds among the HPAMAM molecules play important roles in the self-healing of these hydrogels.36,37 The experiment of self-healing at weak acidic environment was not successful. The OALG coagulated when mixing its solution with the acidified HPAMAM solution (pH = 6–7). No hydrogel could be obtained.
N– bonds. To test that hypothesis, we changed the pH value of the HPAMAM solution to more than 9.0 and mixed it with the OALG solution to fabricate an alkaline hydrogel. It was found that the hydrogel sample could still self-heal (Fig. S5†). It is supposed that the electrostatic interactions and hydrogen bonds between the HPAMAM and OALG molecules, in addition to the hydrogen bonds among the HPAMAM molecules play important roles in the self-healing of these hydrogels.36,37 The experiment of self-healing at weak acidic environment was not successful. The OALG coagulated when mixing its solution with the acidified HPAMAM solution (pH = 6–7). No hydrogel could be obtained.
3.4 pH sensibility, drug release ability and degradation
The acylhydrazone bonds are very sensitive to the pH of the environments.38,39 In addition, the HPAMAM and alginate are both weak polyelectrolytes. It is thus supposed that the HPAMAM/OALG hydrogels should be very sensitive to the pH values. As shown in Fig. 6, the HPAMAM/OALG hydrogel experienced gel–sol–gel transitions with the variation of pH. The hydrogel decomposed and turned into flowing liquids when 1 M HCl solutions were added into the bottle. Apparently, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds were broken, and the electrostatic interactions between HPAMAM and OALG were destroyed too. It led to the collapse of crosslinked structure of hydrogel. Nevertheless, the hydrogel was formed again as proper amount of triethylamine was dropped in, which neutralized the acidic HCl. The electrostatic interactions and hydrogen bonds were re-established, and the –C
N– bonds were broken, and the electrostatic interactions between HPAMAM and OALG were destroyed too. It led to the collapse of crosslinked structure of hydrogel. Nevertheless, the hydrogel was formed again as proper amount of triethylamine was dropped in, which neutralized the acidic HCl. The electrostatic interactions and hydrogen bonds were re-established, and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds were reformed after the aqueous environment shift to basic. Therefore, the networks were rebuilt.
N– bonds were reformed after the aqueous environment shift to basic. Therefore, the networks were rebuilt.
The sensitivity of HPAMAM/OALG hydrogels to pH values suggests that they might possess good drug release ability in acidic circumstance, such as the local environment near the tumour.40 The results from drug release experiments proved such a speculation (Fig. 7). The HPAMAM/OALG hydrogel released the model drugs very quickly at pH = 2. Within 8 hours, the model drugs released nearly 100%. On the contrary, it took about 72 hours for the hydrogel to release the same amount of drugs when the pH was 7.4.
 | ||
| Fig. 7 Release of model drug (rhodamine B) from HPAMAM/OALG hydrogel (Gel-i) under various pH conditions. | ||
The dynamic nature of the acylhydrazone bonds between HPAMAM and OALG will endow the hydrogels good degradation ability. As indicated in Fig. 8, the weight loss of HPAMAM/OALG hydrogel (Gel-i) was as large as 50 wt% in 3 days in weak acidic environment. Interestingly, the degradation rate of HPAMAM/OALG hydrogels could be adjusted through varying the amount of OALG applied, which functioned as crosslinker.
Compared to Gel-i, the Gel-ii showed detained degradation behaviour. In 3 days, the weight loss was only about 25%. The degradation rate of gels was even slower as more OALG were used. For Gel-iii and Gel-iv, the 3 day weight loss was 20% and 15% respectively. It indicated that the degradation of HPAMAM/OALG hydrogels could be finely tuned depending on the jobs.
The differences in degradation behaviour of the hydrogels could be correlated with their morphology. The fracture surface images of freeze-dried HPAMAM/OALG hydrogel (Gel-i and Gel-ii) were shown in Fig. 9. It can be seen that there are lots of pores and channels in Gel-i, while the morphology of Gel-ii is much denser. The cross-section surface is flat, with only a few pores. The denser morphology of Gel-ii suggests that water cannot freely penetrate in and out the hydrogel, which detains the hydrolysis. Due to the different morphology of the hydrogels, their swelling ability was different too (Fig. 10). Gel-ii was of lower swelling ratios compared with Gel-i. The swelling ability of the other two hydrogels (Gel-iii and Gel-iv) was poorer. The equilibrium swelling ratios of Gel-i to Gel-iv were about 7.0, 6.4, 5.6 and 4.8, correspondingly.
 | ||
| Fig. 9 Fracture morphology of freeze-dried HPAMAM/OALG hydrogels. (a) Gel-i, and (b) Gel-ii. The toolbars represents 100 μm. | ||
4. Conclusions
In summary, a kind of fluorescent, self-healing, pH-sensitive and injectable hydrogel was fabricated from HPAMAM and OALG. Hydrogels could be formed very quickly as solutions of the two components were mixed. These hydrogels inherited the unique fluorescent property of the HPAMAMs. There is no need to chemically or physically integrate additional fluorescent reagents into the hydrogel, and thus avoid the possible toxicity from those reagents. It was thought that there were several kinds of dynamic interactions between HPAMAM and OALG, which were also the bases to form hydrogels, that is, the electrostatic force, the hydrogen bonds and the acylhydrazone bonds. All of these dynamic interactions ensured the HPAMAM/OALG hydrogel good self-healing ability, pH-sensitivity and degradation ability. Due to the good biocompatibility of HPAMAM and OALG, the obtained hydrogels are very promising in biomedical applications from tissue engineering to controlled drug release.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 21204016) and the Anhui Provincial Natural Science Foundation (No. 1508085ME107).Notes and references
- L. Yu and J. Ding, Chem. Soc. Rev., 2008, 37, 1473 RSC
.
- N. K. Singh and D. S. Lee, J. Controlled Release, 2014, 193, 214 CrossRef CAS PubMed
.
- K. H. Bae, L.-S. Wang and M. Kurisawa, J. Mater. Chem. B, 2013, 1, 5371 RSC
.
- Y. Zhang, F. Rossi, S. Papa, M. B. Violatto, P. Bigini, M. Sorbona, F. Redaelli, P. Veglianese, J. Hilborn and D. A. Ossipov, Acta Biomater., 2016, 30, 188 CrossRef CAS PubMed
.
- J. Lewandowska-Łańcucka, S. Fiejdasz, Ł. Rodzik, M. Kozieł and M. Nowakowska, Biomed. Mater., 2015, 10, 015020 CrossRef PubMed
.
- R. S. Navath, A. R. Menjoge, H. Dai, R. Romero, S. Kannan and R. M. Kannan, Mol. Pharmaceutics, 2011, 8, 1209 CrossRef CAS PubMed
.
- C. Gao, M. Liu, S. Lü, X. Zhang and H. Duan, J. Mater. Chem. B, 2014, 2, 6823 RSC
.
- L. Li, B. Yan, J. Yang, L. Chen and H. Zeng, Adv. Mater., 2015, 27, 1294 CrossRef CAS PubMed
.
- L. Cai, R. E. Dewi and S. C. Heilshorn, Adv. Funct. Mater., 2015, 25, 1344 CrossRef CAS PubMed
.
- A. K. Gaharwar, R. K. Avery, A. Assmann, A. Paul, G. H. McKinley, A. Khademhosseini and B. D. Olsen, ACS Nano, 2014, 8, 9833 CrossRef CAS PubMed
.
- S. Himmelein, V. Lewe, M. C. A. Stuartb and B. J. Ravoo, Chem. Sci., 2014, 5, 1054 RSC
.
- V. Yesilyurt, M. J. Webber, E. A. Appel, C. Godwin, R. Langer and D. G. Anderson, Adv. Mater., 2016, 28, 86 CrossRef CAS PubMed
.
- N. Huebsch, C. J. Kearney, X. Zhao, J. Kim, C. A. Cezar, Z. Suo and D. J. Mooney, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 9762 CrossRef CAS PubMed
.
- J. Qiu, J. Li, G. Wang, L. Zheng, N. Ren, H. Liu, W. Tang, H. Jiang and Y. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 344 CAS
.
- J. F. Lovell, A. Roxin, K. K. Ng, Q. Qi, J. D. McMullen, R. S. DaCosta and G. Zheng, Biomacromolecules, 2011, 12, 3115 CrossRef CAS PubMed
.
- C. B. Rodell, J. W. MacArthur Jr, S. M. Dorsey, R. J. Wade, L. L. Wang, Y. J. Woo and J. A. Burdick, Adv. Funct. Mater., 2015, 25, 636 CrossRef CAS PubMed
.
- Y. Li, C. Zhou, L. Xu, F. Yao, L. Cen and G. D. Fu, RSC Adv., 2015, 5, 18242 RSC
.
- W. Yang and C.-Y. Pan, Macromol. Rapid Commun., 2009, 30, 2096 CrossRef CAS PubMed
.
- Y.-Z. You, Z.-Q. Yu, M.-M. Cui and C.-Y. Hong, Angew. Chem., 2010, 122, 1117 CrossRef
.
- N. Kuhl, S. Bode, R. K. Bose, J. Vitz, A. Seifert, S. Hoeppener, S. J. Garcia, S. Spange, S. van der Zwaag, M. D. Hager and U. S. Schubert, Adv. Funct. Mater., 2015, 25, 3295 CrossRef CAS
.
- Y. Gao, Q. Luo, S. Qiao, L. Wang, Z. Dong, J. Xu and J. Liu, Angew. Chem., Int. Ed., 2014, 53, 9343 CrossRef CAS PubMed
.
- D. D. McKinnon, D. W. Domaille, J. N. Cha and K. S. Anseth, Adv. Mater., 2014, 26, 865 CrossRef CAS PubMed
.
- D. E. Whitaker, C. S. Mahon and D. A. Fulton, Angew. Chem., 2013, 125, 990 CrossRef
.
- W. Hao, S. Ding, L. Zhang, W. Liu and W. Yang, ChemPlusChem, 2014, 79, 211 CrossRef CAS
.
- W. Hao, L. Zhang, X. Wang, J. Wang, Z. Hu and W. Yang, RSC Adv., 2016, 6, 1415 RSC
.
- W. Hao, X. Wang, S. Ding, Y. Cao, H. Zhang and W. Yang, RSC Adv., 2015, 5, 86861 RSC
.
- C. G. Gomez, M. Rinaudo and M. A. Villar, Carbohydr. Polym., 2007, 67, 296 CrossRef CAS
.
- L. Li, N. Wang, X. Jin, R. Deng, S. Nie, L. Sun, Q. Wu, Y. Wei and C. Gong, Biomaterials, 2014, 35, 3903 CrossRef CAS PubMed
.
- C. D. Hermann, D. S. Wilson, K. A. Lawrence, X. Ning, R. Olivares-Navarrete, J. K. Williams, R. E. Guldberg, N. Murthy, Z. Schwartz and B. D. Boyan, Biomaterials, 2014, 35, 9698 CrossRef CAS PubMed
.
- Y. Jia, J. Fei, Y. Cui, Y. Yang, L. Gao and J. Li, Chem. Commun., 2011, 47, 1175 RSC
.
- X. Ma, J. Deng, Y. Du, X. Li, D. Fan, C. Zhu, J. Hui, P. Ma and W. Xue, J. Mater. Chem. B, 2014, 2, 2749 RSC
.
- D. Wang and T. Imae, J. Am. Chem. Soc., 2004, 126, 13204 CrossRef CAS PubMed
.
- S. Zhu, Y. Song, J. Shao, X. Zhao and B. Yang, Angew. Chem., Int. Ed., 2015, 54, 14626 CrossRef CAS PubMed
.
- G. Deng, F. Li, H. Yu, F. Liu, C. Liu, W. Sun, H. Jiang and Y. Chen, ACS Macro Lett., 2012, 1, 275 CrossRef CAS
.
- Y. Zhang, B. Yang, X. Zhang, L. Xu, L. Tao, S. Li and Y. Wei, Chem. Commun., 2012, 48, 9305 RSC
.
- R. Peng, Y. Yu, S. Chen, Y. Yang and Y. Tang, RSC Adv., 2014, 4, 35149 RSC
.
- F. Luo, T. L. Sun, T. Nakajima, T. Kurokawa, Y. Zhao, K. Sato, A. B. Ihsan, X. Li, H. Guo and J. P. Gong, Adv. Mater., 2015, 27, 2722 CrossRef CAS PubMed
.
- Y. Zhang, L. Tao, S. Li and Y. Wei, Biomacromolecules, 2011, 12, 2894 CrossRef CAS PubMed
.
- G. Deng, C. Tang, F. Li, H. Jiang and Y. Chen, Macromolecules, 2010, 43, 1191 CrossRef CAS
.
- D. Das and S. Pal, RSC Adv., 2015, 5, 25014 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02366e |
| This journal is © The Royal Society of Chemistry 2016 |