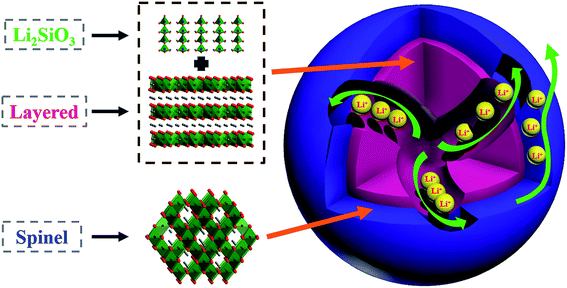
Li+-conductive Li2SiO3 stabilized Li-rich layered oxide with an in situ formed spinel nano-coating layer: toward enhanced electrochemical performance for lithium-ion batteries†
Mingquan Xua,
Qingwang Liana,
Yuxin Wua,
Cheng Maa,
Pengfei Tana,
Qingbing Xiaa,
Jinfang Zhanga,
Douglas G. Iveyb and
Weifeng Wei *a
*a
aState Key Laboratory of Powder Metallurgy, Central South University, Changsha, 410083, P. R. China. E-mail: weifengwei@csu.edu.cn; Fax: +86 73188877876; Tel: +86 83188877876
bDepartment of Chemical and Materials Engineering, University of Alberta, Edmonton, AB T6G 2V4, Canada
First published on 1st April 2016
Abstract
A novel heterostructured cathode material, comprised of a core of Li-rich layered (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) oxides integrated with Li+-conductive Li2SiO3 and an in situ formed spinel (Fd
m) oxides integrated with Li+-conductive Li2SiO3 and an in situ formed spinel (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) nano-coating layer, has been successfully synthesized. The ionic conductor Li2SiO3 in the bulk facilitates the Li+ intercalation/deintercalation process and stabilizes the layered structure. This is coupled with the fast 3D Li+ diffusion channels of an electrochemically active spinel coating layer, leading to superior rate capability (94 mA h g−1 at 10C) and excellent cycling stability in the heterostructured cathode material. This synergistic strategy may provide some new directions for synthesizing high-performance Li-ion battery cathode materials.
m) nano-coating layer, has been successfully synthesized. The ionic conductor Li2SiO3 in the bulk facilitates the Li+ intercalation/deintercalation process and stabilizes the layered structure. This is coupled with the fast 3D Li+ diffusion channels of an electrochemically active spinel coating layer, leading to superior rate capability (94 mA h g−1 at 10C) and excellent cycling stability in the heterostructured cathode material. This synergistic strategy may provide some new directions for synthesizing high-performance Li-ion battery cathode materials.
Introduction
With the rapid development of portable electronic devices, electric vehicles, and renewable energy storage in smart grids, high-performance rechargeable lithium-ion batteries (LIBs) are urgently needed.1–4 However, commercialized cathode materials, such as LiCoO2, LiFePO4, and LiMn1/3Ni1/3Co1/3O3, cannot satisfy the growing demand for high energy- and power-density LIBs, due to their limited theoretical capacities (<200 mA h g−1).5–9 Recently, Li-rich layered oxides, with the general formula of xLi2MnO3·(1 − x)LiTMO2 (TM represents Mn, Ni, Co, etc.), have been considered as one of the promising candidate cathode materials for the next-generation of advanced LIBs, due to their encouraging features such as higher specific capacity (exceeding 250 mA h g−1), higher operating voltage (over 4.5 V vs. Li+/Li) and lower cost.10,11Despite these advantages, there are still some crucial drawbacks hindering Li-rich layered oxides from implementation in commercial applications. For instance, structural collapse on cycling is one major deficiency of Li-rich layered oxides, which has been verified to be associated with the release of Li2O on charging at high potential and the inevitable layered-to-spinel structural transformation, resulting in limited cycle performance.12–14 Li-rich layered oxides also suffer from sluggish electrochemical kinetics resulting from their intrinsic two-dimensional Li+ diffusion tunnels, leading to poor rate capability.11,15
In recent decades, doping methods and surface modification have been widely employed to address these challenges. Cationic doping, by substituting Mn, Ni, and Co with other fixed valence state cations (such as Al, Sn, Ru, etc.),16–18 substantially improves the cyclic durability and rate performance by weakening the transition metal–oxygen bonds and promoting Li+ diffusion. Nevertheless, atomic substitution only changes the local chemical environment of certain atoms rather than altering the intrinsic 2D Li+ transport pathways in the bulk. As another method, surface coating has been proved an effective approach to protect electrode materials.19–21 Concerning Li-rich layered oxides, the formation of coating layer (such as metal oxides, metal phosphates, and metal fluorides) on the cathode surface,22–26 to some extent, prevents cathode materials from being involved in side reactions with the electrolyte under high voltage and stabilizes the surface structure. However, most of the coating materials are generally electrochemically inactive, leading to improved cycling stability at the expense of reduced specific capacity. Furthermore, conventional coating approaches rarely solve the instability of the Li2MnO3 phase in Li-rich layered oxides, which was reported to be a major cause for the deterioration of their electrochemical performance.27,28
Herein, we integrated a structurally compatible unit into the Li-rich layered oxides to improve their structural stability and enhance the electrochemical performance. Simultaneously, an electrochemically active layer in situ formed on the surface, which offsets the capacity loss due to the integration of the inactive component, as shown in Fig. 1. This heterostructured cathode material possesses the following attributes: (1) different from atomic doping, orthorhombic Li2SiO3, a layered material similar to Li2MnO3, is regarded as an ideal structural unit to stabilize Li-rich layered oxides, due to stronger Si–O bonds (ΔHf (Si–O) = 798 kJ mol−1) than TM–O bonds (e.g. ΔHf (Mn–O) = 402 kJ mol−1).29 Additionally, Li2SiO3, possessing 3D Li-ion diffusion pathways, acts as a Li+ transport accelerator in the layered bulk. (2) The spinel (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) oxides, characteristic of the same oxygen arrangement with the layered (R
m) oxides, characteristic of the same oxygen arrangement with the layered (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) structure, also exhibit excellent structural compatibility with the layered bulk as a coating layer, and reinforce the Li+ migration between the bulk and the electrolyte due to their 3D Li+ transport channels.30 This unique cathode material with such heterostructure yields superior rate capability and favorable cycling performance.
m) structure, also exhibit excellent structural compatibility with the layered bulk as a coating layer, and reinforce the Li+ migration between the bulk and the electrolyte due to their 3D Li+ transport channels.30 This unique cathode material with such heterostructure yields superior rate capability and favorable cycling performance.
Experimental section
Synthesis of the pristine Li1.2Mn0.54Ni0.13Co0.13O2 and Li2SiO3 layered materials
The pristine Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 material was synthesized via a sol–gel process. Stoichiometric amounts of lithium acetate dihydrate, manganese acetate tetrahydrate, nickel acetate tetrahydrate, and cobalt acetate tetrahydrate were dissolved in distilled water while stirring (with 5 wt% of excess Li to compensate for the loss of lithium salt during calcination). Then a certain amount of citric acid was added into the solution as a chelating agent (citric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal ion molar ratio = 1
metal ion molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The solution was evaporated at 80 °C, leading to forming a colloidal gel, which was then dried at 120 °C in a vacuum oven for 24 h. After grinding, the powders were precalcined at 500 °C for 5 h to remove the organic components and then calcined at 900 °C in a muffle furnace to obtain the target product, which is named pristine. For the preparation of the 3% Li2SiO3·97% Li1.2 Mn0.54Ni0.13Co0.13O2 material, the synthetic conditions remained unchanged except that the starting materials contained lithium acetate dihydrate (5% excess), manganese acetate tetrahydrate, nickel acetate tetrahydrate, cobalt acetate tetrahydrate and tetraethyl orthosilicate (TEOS) (as the silica source) with the stoichiometric molar ratio. The as-prepared material is labeled as LS.
1). The solution was evaporated at 80 °C, leading to forming a colloidal gel, which was then dried at 120 °C in a vacuum oven for 24 h. After grinding, the powders were precalcined at 500 °C for 5 h to remove the organic components and then calcined at 900 °C in a muffle furnace to obtain the target product, which is named pristine. For the preparation of the 3% Li2SiO3·97% Li1.2 Mn0.54Ni0.13Co0.13O2 material, the synthetic conditions remained unchanged except that the starting materials contained lithium acetate dihydrate (5% excess), manganese acetate tetrahydrate, nickel acetate tetrahydrate, cobalt acetate tetrahydrate and tetraethyl orthosilicate (TEOS) (as the silica source) with the stoichiometric molar ratio. The as-prepared material is labeled as LS.
Synthesis of the Li2SiO3 layered@spinel heterostructured material
400 mg of the LS sample was dispersed in 120 mL of 10 mM tris-buffer aqueous solution (pH ∼ 8.5) by sonication prior to the addition of 60 mg of dopamine. After polymerizing while continuously stirring for 6 h, the product was collected by filtration and then dried under vacuum at 80 °C. The heating treatment was carried out at 550 °C for 10 min and then the sample was cooled to room temperature in air. The treated material is denoted as LS@S.Materials characterization
The phase and crystal structure of the synthesized materials were characterized by powder X-ray diffraction (XRD) using a Rigaku D/Max-2500 Diffractometer with Cu Kα radiation (λ = 0.15405 nm) under steps of 0.02° at a constant counting time of 2 s. The Rietveld refinement is performed with General Structure Analysis System (GSAS) software.31 The morphology of the samples was observed using a Nova Nano SEM230 field emission scanning electron microscope (SEM) and a FEI Titan G2 60-300 transmission electron microscope (TEM). Energy dispersive X-ray (EDX) element mapping was recorded by a FEI Titan G2 60-300 TEM with a Super-X EDX detector system operated at 300 kV. Chemical state analysis of elements was carried out by X-ray photoelectron spectroscopy (XPS) using an ESCALAB 250Xi X-ray photoelectron spectrometer. Etching with Ar+ was used to determine depth profiles of Mn, Ni, and Co at an estimated rate of 5.4 nm min−1. All XPS spectra were calibrated against the C 1s line at 284.8 eV.Electrochemical testing
To prepare the working electrodes, a mixture of the active material, acetylene black, and polyvinylidene fluorides (PVDF) with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in N-methyl pyrrolidinone (NMP) was pasted on Al foil and then dried at 110 °C for 12 h under vacuum. Geometrical area of the electrodes used was about 1.13 cm2 (12 mm in diameter) and the active material mass was around 2–4 mg. The assembly of CR2025-type coin cells was conducted in an Ar-filled glove box with a Li metal foil as the counter electrode, a Celgard-2400 porous polypropylene as the separator and a solution of 1 M LiPF6 in EC–DMC (1
1 in N-methyl pyrrolidinone (NMP) was pasted on Al foil and then dried at 110 °C for 12 h under vacuum. Geometrical area of the electrodes used was about 1.13 cm2 (12 mm in diameter) and the active material mass was around 2–4 mg. The assembly of CR2025-type coin cells was conducted in an Ar-filled glove box with a Li metal foil as the counter electrode, a Celgard-2400 porous polypropylene as the separator and a solution of 1 M LiPF6 in EC–DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v%) as the electrolyte. The galvanostatic charge and discharge behaviors of the cells were performed using a battery testing system (LANHE CT2001A, Wuhan LAND Electronics Co., P. R. China) between 2.0 V and 4.8 V at different current densities. Cyclic voltammetry (CV) was carried out with a Princeton PARSTAT 4000 (AMETEK Co. Ltd.) electrochemistry workstation at a scan rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS) data were collected in a frequency range from 100 mHz to 100 kHz with an AC amplitude 5 mV on the P4000 instrument.
1, v%) as the electrolyte. The galvanostatic charge and discharge behaviors of the cells were performed using a battery testing system (LANHE CT2001A, Wuhan LAND Electronics Co., P. R. China) between 2.0 V and 4.8 V at different current densities. Cyclic voltammetry (CV) was carried out with a Princeton PARSTAT 4000 (AMETEK Co. Ltd.) electrochemistry workstation at a scan rate of 0.1 mV s−1. Electrochemical impedance spectroscopy (EIS) data were collected in a frequency range from 100 mHz to 100 kHz with an AC amplitude 5 mV on the P4000 instrument.
Results and discussion
Morphology and structural characterizations
Fig. 2 shows SEM images of the pristine, LS and LS@S samples. All the powders are comprised of irregular polyhedrons with a similar morphology. The crystalline structure of the pristine, LS, and LS@S materials were revealed by XRD, as shown in Fig. 3. The main peaks for all samples can be indexed to a layered α-NaFeO2 structure with R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry. Additional weak peaks, appearing in the range of 20–25°, are considered as reflections from Li and Mn ordering within the TM layer of the monoclinic (C2/m) Li2MnO3 regions, which is a typical feature of Li-rich layered oxides.10,32 No impurity peaks corresponding to silicon-related compounds were observed in the XRD patterns, because of the relatively low amount of loading. When the Li2SiO3 content in the LS material was increased to 6%, weak reflections of Li2SiO3 appeared (as shown in Fig. S1†). It should be noted that, after the post-heat treatment at 550 °C, a distinct satellite hump was observed in the XRD pattern of the LS@S sample, as indicated by the arrow in Fig. 3, which can be assigned to the X-ray reflections of the spinel structure (Fd
m symmetry. Additional weak peaks, appearing in the range of 20–25°, are considered as reflections from Li and Mn ordering within the TM layer of the monoclinic (C2/m) Li2MnO3 regions, which is a typical feature of Li-rich layered oxides.10,32 No impurity peaks corresponding to silicon-related compounds were observed in the XRD patterns, because of the relatively low amount of loading. When the Li2SiO3 content in the LS material was increased to 6%, weak reflections of Li2SiO3 appeared (as shown in Fig. S1†). It should be noted that, after the post-heat treatment at 550 °C, a distinct satellite hump was observed in the XRD pattern of the LS@S sample, as indicated by the arrow in Fig. 3, which can be assigned to the X-ray reflections of the spinel structure (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m).33–35 Rietveld refinement results (as summarized in Table 1) showed that, after introducing Li2SiO3, c lattice parameter values exhibited a slightly decrease. This is probably due to higher bonding energy of Si–O than M–O, because c represents the interspacing of TM layers.17 Here, LS sample had the lowest Li/Ni disordering degree (1.01%), indicating that introducing Li2SiO3 into Li-rich layered oxides helped to form more ordered layered structure. A relatively increased χ value for LS@S was caused by metal ion migration during in situ phase transformation. The change in χ values is in accordance with that for the I(003)/I(104) ratios. The phase composition was also achieved by Rietveld refinement, which approximately accorded with the nominal composition.
m).33–35 Rietveld refinement results (as summarized in Table 1) showed that, after introducing Li2SiO3, c lattice parameter values exhibited a slightly decrease. This is probably due to higher bonding energy of Si–O than M–O, because c represents the interspacing of TM layers.17 Here, LS sample had the lowest Li/Ni disordering degree (1.01%), indicating that introducing Li2SiO3 into Li-rich layered oxides helped to form more ordered layered structure. A relatively increased χ value for LS@S was caused by metal ion migration during in situ phase transformation. The change in χ values is in accordance with that for the I(003)/I(104) ratios. The phase composition was also achieved by Rietveld refinement, which approximately accorded with the nominal composition.
| Sample | a/Å | c/Å | I(003)/I(104) | Li2MnO3/% | χ |
|---|---|---|---|---|---|
| a Note: χ represents the degree of Li/Ni disordering. | |||||
| Pristine | 2.8583 | 14.2516 | 1.085 | 46.16% | 2.11% |
| LS | 2.8547 | 14.2267 | 1.207 | 44.25% | 1.01% |
| LS@S | 2.8598 | 14.2219 | 1.113 | 44.79% | 1.79% |
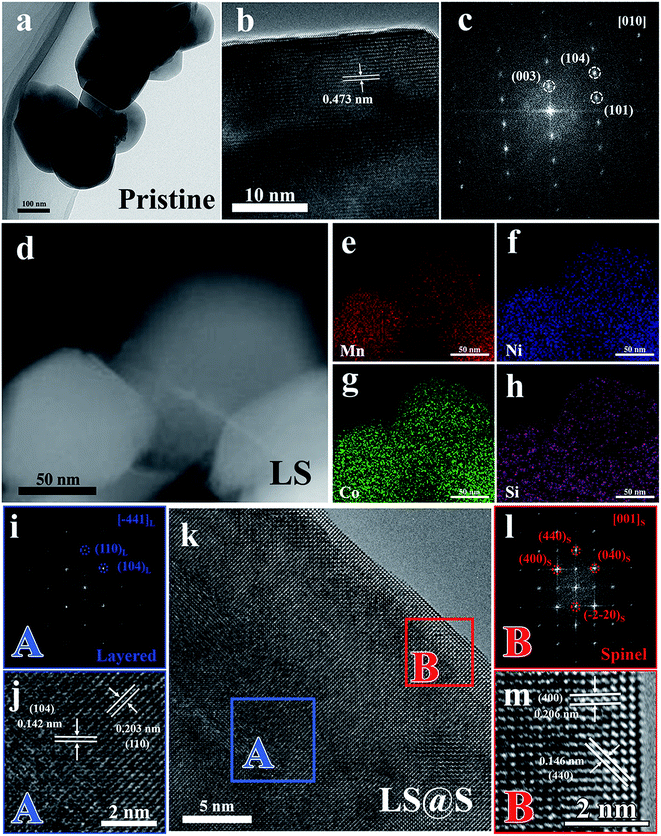
To further investigate the microstructure of the materials in detail, the pristine, LS, and LS@S samples were characterized using TEM. The pristine and LS samples both consist of primary particles about 200 nm in size (Fig. 4a and d). The high resolution TEM (HRTEM) micrograph (Fig. 4b), taken from the pristine sample, exhibits clear lattice fringes with a d spacing equal to 0.475 nm, which corresponds to the (003) planes of the layered structure (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). Fig. 4c shows the fast Fourier transformation (FFT) pattern derived from Fig. 4b, and the diffraction spots can be indexed to the (003), (101), and (104) planes of the layered structure, which is consistent with the R
m). Fig. 4c shows the fast Fourier transformation (FFT) pattern derived from Fig. 4b, and the diffraction spots can be indexed to the (003), (101), and (104) planes of the layered structure, which is consistent with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group along [010] zone axis. For the LS material, a high-angle annular dark-field scanning TEM (HAADF-STEM) image is displayed in Fig. 4d. Elemental mapping for Mn, Ni, Co, and Si, as shown in Fig. 4e–h, demonstrates that the elements are distributed uniformly through the LS material. In combination with the XRD results, we can surmise that the LS sample is a homogeneous composition of Li2SiO3 and Li-rich layered oxides. Fig. 4k shows a HRTEM image of the LS@S material. Based on the detailed HRTEM image and corresponding FFT pattern (shown in Fig. 4i and j), the bulk (domain A in Fig. 4k) remains the typical layered structure with two interplanar spacings of 0.203 nm and 0.142 nm related to the (104) and (110) planes of the R
m space group along [010] zone axis. For the LS material, a high-angle annular dark-field scanning TEM (HAADF-STEM) image is displayed in Fig. 4d. Elemental mapping for Mn, Ni, Co, and Si, as shown in Fig. 4e–h, demonstrates that the elements are distributed uniformly through the LS material. In combination with the XRD results, we can surmise that the LS sample is a homogeneous composition of Li2SiO3 and Li-rich layered oxides. Fig. 4k shows a HRTEM image of the LS@S material. Based on the detailed HRTEM image and corresponding FFT pattern (shown in Fig. 4i and j), the bulk (domain A in Fig. 4k) remains the typical layered structure with two interplanar spacings of 0.203 nm and 0.142 nm related to the (104) and (110) planes of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. Distinct from the layered bulk, a nano-coating layer with a thickness of 3–5 nm appears along the layered oxide surface in Fig. 4k. From the FFT pattern (Fig. 4l) taken from domain B in Fig. 4k, the diffraction spots (as marked by red cycles) can be indexed to the (400) and (040) planes along the [001] zone axis of the cubic spinel phase (Fd
m space group. Distinct from the layered bulk, a nano-coating layer with a thickness of 3–5 nm appears along the layered oxide surface in Fig. 4k. From the FFT pattern (Fig. 4l) taken from domain B in Fig. 4k, the diffraction spots (as marked by red cycles) can be indexed to the (400) and (040) planes along the [001] zone axis of the cubic spinel phase (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The enlarged HRTEM image (Fig. 4m) reveals that the coating layer has lattice spacings of 0.206 nm and 0.146 nm, which are in accordance with (040) and (440) planes of the cubic spinel structure (Fd
m). The enlarged HRTEM image (Fig. 4m) reveals that the coating layer has lattice spacings of 0.206 nm and 0.146 nm, which are in accordance with (040) and (440) planes of the cubic spinel structure (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m), respectively. It should be noted that, there exists no clear interface between the spinel phase in the surface and the layered phase in the bulk, presumably because the newly formed cubic spinel phase has the same oxygen close-packed stacking with the hexagonal layered bulk, indicating the spinel nano-coating layer exhibits good structural compatibility with the layered bulk.36 Consequently, the LS@S material is composed of a layered bulk integrating Li+-conductive Li2SiO3 and an in situ formed spinel nano-coating layer with 3D Li+ diffusion channels.
m), respectively. It should be noted that, there exists no clear interface between the spinel phase in the surface and the layered phase in the bulk, presumably because the newly formed cubic spinel phase has the same oxygen close-packed stacking with the hexagonal layered bulk, indicating the spinel nano-coating layer exhibits good structural compatibility with the layered bulk.36 Consequently, the LS@S material is composed of a layered bulk integrating Li+-conductive Li2SiO3 and an in situ formed spinel nano-coating layer with 3D Li+ diffusion channels.
X-ray photoelectron spectroscopy (XPS) was used to analyze the chemical states of the transition metal elements. Typical XPS spectra for Mn 2p, Ni 2p, and Co 2p of the pristine and LS material samples are compared in Fig. 5a–c. For the pristine sample, the 2p3/2 peaks of Mn, Ni, and Co at binding energies of 642.3, 855.2, and 780.3 eV, which are in good agreement with those of Mn4+, Ni2+, and Co3+, respectively.19,37,38 For the LS sample, the binding energies for the Mn 2p, Ni 2p, and Co 2p are retained, suggesting that integrating Li2SiO3, as a structural unit, into the Li-rich layered oxides has little effect on the local chemical environment of transition metal elements. Fig. 5d shows that the 2s peak of Si at 153.1 eV corresponds to a charge of 4+, which is in accordance with the valence of Si in Li2SiO3.39,40
 | ||
| Fig. 5 XPS spectra for Mn 2p (a), Ni 2p (b), Co 2p (c), and Si 2s (d) of the pristine and LS samples. | ||
To further investigate any compositional variation within the LS and LS@S samples from the surface to bulk, XPS depth profiling was conducted, as shown in Fig. 6a and b. There is little change in the atomic ratios for Mn, Ni, and Co from the surface to bulk of the LS sample. However, in the LS@S sample, the Mn composition in the surface region is higher than that in the bulk and Ni and Co compositions are lower in the surface relative to the bulk (as marked by the dotted box). This variation in composition suggests that substantial ion rearrangement occurred during the post-heating process. As shown in Fig. 6c, the O 1s core spectrum consists of two peaks corresponding to the Li–O bond and the TM–O bond, respectively.41,42 Compared with the LS sample, the Li–O peak is substantially lower in the LS@S sample. Meanwhile, the Li 1s spectrum (Fig. 6d) also confirms that the amount of Li in the LS@S sample is lower, due to its weaker intensity. These results reveal the formation of Li vacancies during the post-heating treatment, which may be, in some way, analogous to the process of Li2O extraction during chemical activation. During the annealing process, the presence of Li vacancies facilitates cation mobility. The transition metal ions originally located in 3b octahedral sites migrate into the empty Li sites in the Li layer at high temperature, which induces the layered-to-spinel structural transformation and in situ forms the spinel nano-coating layer.43
Electrochemical performance
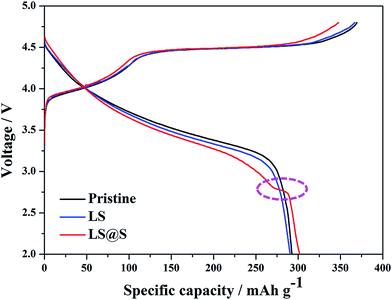
Typical initial charge–discharge profiles for the pristine, LS, and LS@S samples at 0.1C (1C = 250 mA h g−1) in the voltage window of 2.0–4.8 V are shown in Fig. 7. It is clear that the charging curves, for all samples, can be divided into two distinguishable regions. There are, a smoothly sloping stage below 4.5 V related to the Ni2+ → Ni4+ and Co3+ → Co4+ oxidizing reactions and a long plateau around 4.5 V corresponding to the extraction of Li from the Li2MnO3 component accompanied with the simultaneous release of oxygen and structural rearrangement.13,44 The charge–discharge curves of the pristine and LS sample are similar except that the latter exhibits slightly lower charge and discharge capacities, since Li2SiO3 is electrochemically inert during the charge–discharge process. In comparison with the pristine and LS materials, the LS@S sample has a decreased charge capacity due to the shortened voltage plateau around 4.5 V. This suggests that, during the ion rearrangement, the partial Li2MnO3 was pre-activated for the LS@S material. Furthermore, a short discharge plateau appears around 2.7 V, which refers to the intercalation of Li+ into the spinel structure,26,45,46 resulting in a higher discharge capacity of 302 mA h g−1. Thus, the LS@S sample exhibits the highest initial coulombic efficiency of 86.8% with the lowest irreversible capacity loss of 46 mA h g−1 (as tabulated in Table 2). The EIS data also indicate (as shown in Fig. S2†) that the LS@S material has the smallest value of the surface charge-transfer resistance (Rct), which contributes a high rate capability. In addition, from the CV curves of the LS@S sample in the first three cycles (Fig. S3†), redox couples below 3 V are observed (as marked by the dotted box), which corresponds to the lithiation/delithiation mechanism of the spinel structure, suggesting that the in situ formed spinel layer also participates in the electrochemical reactions.| Charge capacity (mA h g−1) | Discharge capacity (mA h g−1) | Coulombic efficiency (%) | |
|---|---|---|---|
| Pristine | 369 | 293 | 79.4 |
| LS | 367 | 291 | 79.3 |
| LS@S | 348 | 302 | 86.8 |
The rate performance of the pristine, LS, and LS@S samples at different current densities from 0.2C to 10C was studied, and the results are shown in Fig. 8. For the pristine sample, the discharge capacity decays dramatically with increasing current density. When the rate is increased to 2C, the pristine sample only discharges half of the capacity of the LS material, and it delivers nearly no discharge capacity at a rate of 8C. The intrinsic two-dimensional Li+ diffusion channels in the Li-rich layered oxides lead to the poor rate capability of the pristine material, which limits Li+ migration in the bulk.11 In contrast, the LS sample exhibits better rate capability, with discharge capacities of 82 mA h g−1 and 49 mA h g−1 at 8C and 10C, respectively. The enhanced rate capability suggests that integrating Li+-conductive Li2SiO3 into the layered bulk facilitates the Li+ intercalation/deintercalation process by supplying three-dimensional Li+ migration pathways in the bulk.47 Furthermore, the LS@S sample shows better rate capability than the other two materials. Not only is the discharge capacity higher at low rates, but as the current density increases, the capacity differences becomes more marked. For instance, when discharging at 2C, the LS@S sample delivers the highest capacity of 227 mA h g−1, whereas the pristine and LS sample have lower capacities of 186 mA h g−1 and 104 mA h g−1, respectively. Even at 10C, the LS@S electrode still has a discharge capacity of 94 mA h g−1, which is approximately twice that of the LS sample at the identical rate and nearly the same capacity of the pristine sample at 2C. This indicates that the spinel nano-coating layer with 3D Li+ diffusion channels expedites Li+ transport between the electrode and electrolyte. This is coordinated with the Li+-conductive Li2SiO3 component in the bulk and enhances the intrinsic sluggish kinetics of Li-rich layered oxides.
In order to investigate the effect of structural integration and surface modification on cycling capability, the cells were tested between 2.0 V and 4.8 V at a current density of 1C after activation at 0.1C for 2 cycles, as shown in Fig. 9a. The LS material exhibits a better capacity retention of 80.6% with a higher discharge capacity of 169 mA h g−1 after 100 cycles, while the pristine sample fades to 134 mA h g−1 with a capacity retention of 67.1%. Fig. 9b shows that the pristine material undergoes a drastic working voltage decay and severe capacity loss during cycling, indicating that the Li-rich layered oxide suffers from an inevitably continuous layered-to-spinel structural transformation.14,48 In comparison, the voltage fading of the LS sample has been effectively mitigated (as shown in Fig. 9c) via integration of Li2SiO3 as a structural unit into the layered bulk, suggesting that Li2SiO3 plays a crucial role in stabilizing the layered structure in the electrochemical process due to stronger Si–O bonds (ΔHf (Si–O) = 798 kJ mol−1) than TM–O bonds (e.g., ΔHf (Mn–O) = 402 kJ mol−1).29 For the LS@S sample (Fig. 9d), a higher initial discharge capacity of 225 mA h g−1 at 1C is achieved, and even after 100 cycles, it still delivers a higher discharge capacity than the LS sample, which benefits from the electrochemical activity below 3 V of the newly formed spinel phase. The enhanced discharge capacity and better cycling stability for the LS@S sample is attributed to the synergistic effect of Li+-conductive Li2SiO3 in the layered bulk and the electrochemically active spinel nano-coating layer.
Conclusions
In this work, a synergistic strategy has been employed to synthesize a novel Li-rich heterostructured cathode material with excellent structural stability and high discharge capacity for Li-ion batteries. An electrochemically active spinel nano-coating layer with 3D Li+ migration channels expedites Li+ transfer between electrode/electrolyte interfaces, which forms on the surface of the layered bulk via in situ structural rearrangement. In addition, Li+-conductive Li2SiO3 is integrated homogeneously into the layered bulk by a sol–gel method, facilitating Li+ migration in the bulk and stabilizing the layered structure. The favorable electrochemical performance, including reduced initial irreversible capacity, enhanced cycling stability, and improved discharge capacity, demonstrate the synergistic effect of Li+-conductive Li2SiO3 in the bulk and electrochemically active spinel nano-coating layer. This may provide some technical insights to further negate the shortcomings of the Li-rich layered oxides, synthesizing high-performance cathode materials for next-generation Li-ion batteries.Acknowledgements
This work was supported by the Recruitment Program of Global Youth Experts, the National Natural Science Foundation of China (51304248), the Program for New Century Excellent Talents in University (NCET-11-0525), the Program for Shenghua Overseas Talents from Central South University (CSU), Grants from the Project of Innovation-driven Plan in Central South University and the State Key Laboratory of Powder Metallurgy at Central South University.References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- H. C. Yu, C. Ling, J. Bhattacharya, J. C. Thomas, K. Thornton and A. Van der Ven, Energy Environ. Sci., 2014, 7, 1760–1768 CAS.
- Y. G. Guo, J. S. Hu and L. J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS.
- M. M. Thackeray, C. Wolverton and E. D. Isaacs, Energy Environ. Sci., 2012, 5, 7854–7863 CAS.
- N. S. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y. K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994–10024 CrossRef CAS PubMed.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, J. Power Sources, 2006, 159, 263–267 CrossRef CAS.
- X. Zhao, M. V. Reddy, H. Liu, G. V. Subba Rao and B. V. R. Chowdari, RSC Adv., 2014, 4, 24538–24543 RSC.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed.
- M. M. Thackeray, S. H. Kang, C. S. Johnson, J. T. Vaughey, R. Benedek and S. A. Hackney, J. Mater. Chem., 2007, 17, 3112–3125 RSC.
- H. Yu and H. Zhou, J. Phys. Chem. Lett., 2013, 4, 1268–1280 CrossRef CAS PubMed.
- N. Tran, L. Croguennec, M. Ménétrier, F. Weill, P. Biensan, C. Jordy and C. Delmas, Chem. Mater., 2008, 20, 4815–4825 CrossRef CAS.
- S. Hy, F. Felix, J. Rick, W. N. Su and B. J. Hwang, J. Am. Chem. Soc., 2014, 136, 999–1007 CrossRef CAS PubMed.
- M. Gu, I. Belharouak, J. Zheng, H. Wu, J. Xiao, A. Genc, K. Amine, S. Thevuthasan, D. R. Baer, J. G. Zhang, N. D. Browning, J. Liu and C. Wang, ACS Nano, 2012, 7, 760–767 CrossRef PubMed.
- H. Yu, Y. Wang, D. Asakura, E. Hosono, T. Zhang and H. Zhou, RSC Adv., 2012, 2, 8797–8807 RSC.
- C. C. Wang, Y. C. Lin and P. H. Chou, RSC Adv., 2015, 5, 68919–68928 RSC.
- Q. Q. Qiao, L. Qin, G. R. Li, Y. L. Wang and X. P. Gao, J. Mater. Chem. A, 2015, 3, 17627–17634 CAS.
- J. C. Knight, P. Nandakumar, W. H. Kan and A. Manthiram, J. Mater. Chem. A, 2015, 3, 2006–2011 CAS.
- K. S. Tan, M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, J. Power Sources, 2005, 141, 129–142 CrossRef CAS.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Electrochim. Acta, 2005, 50, 3375–3382 CrossRef CAS.
- M. Prabu, M. V. Reddy, S. Selvasekarapandian, S. Admas, K. P. Loh, G. V. S. Rao and B. V. R. Chowdari, J. Electrochem. Soc., 2013, 160, A3144–A3147 CrossRef CAS.
- S. Guo, H. Yu, P. Liu, X. Liu, D. Li, M. Chen, M. Ishida and H. Zhou, J. Mater. Chem. A, 2014, 2, 4422–4428 CAS.
- X. Zhang, I. Belharouak, L. Li, Y. Lei, J. W. Elam, A. Nie, X. Chen, R. S. Yassar and R. L. Axelbaum, Adv. Energy Mater., 2013, 3, 1299–1307 CrossRef CAS.
- M. S. Park, J. W. Lee, W. Choi, D. Im, S. G. Doo and K. S. Park, J. Mater. Chem., 2010, 20, 7208–7213 RSC.
- Q. Y. Wang, J. Liu, A. V. Murugan and A. Manthiram, J. Mater. Chem., 2009, 19, 4965–4972 RSC.
- Y. K. Sun, M. J. Lee, C. S. Yoon, J. Hassoun, K. Amine and B. Scrosati, Adv. Mater., 2012, 24, 1192–1196 CrossRef CAS PubMed.
- M. Jiang, B. Key, Y. S. Meng and C. P. Grey, Chem. Mater., 2009, 21, 2733–2745 CrossRef CAS.
- A. Boulineau, L. Simonin, J. F. Colin, C. Bourbon and S. Patoux, Nano Lett., 2013, 13, 3857–3863 CrossRef CAS PubMed.
- N. A. Lange and J. A. Dean, Lange's Handbook of Chemistry, McGraw-Hill, 1973 Search PubMed.
- A. Kraytsberg and Y. Ein Eli, Adv. Energy Mater., 2012, 2, 922–939 CrossRef CAS.
- B. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- A. R. Armstrong, M. Holzapfel, P. Novak, C. S. Johnson, S. H. Kang, M. M. Thackeray and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 8694–8698 CrossRef CAS PubMed.
- D. Wang, I. Belharouak, G. Zhou and K. Amine, Adv. Funct. Mater., 2013, 23, 1070–1075 CrossRef CAS.
- I. Belharouak, G. M. Koenig Jr, J. Ma, D. P. Wang and K. Amine, Electrochem. Commun., 2011, 13, 232–236 CrossRef CAS.
- D. Kim, G. Sandi, J. R. Croy, K. G. Gallagher, S. H. Kang, E. Lee, M. D. Slater, C. S. Johnson and M. M. Thackeray, J. Electrochem. Soc., 2013, 160, A31–A38 CrossRef CAS.
- M. M. Thackeray, C. S. Johnson, J. T. Vaughey, N. Li and S. A. Hackney, J. Mater. Chem., 2005, 15, 2257–2267 RSC.
- Q. Fu, F. Du, X. Bian, Y. Wang, X. Yan, Y. Zhang, K. Zhu, G. Chen, C. Wang and Y. Wei, J. Mater. Chem. A, 2014, 2, 7555–7562 CAS.
- M. V. Reddy, Y. Xu, V. Rajarajan, T. Ouyang and B. V. R. Chowdari, ACS Sustainable Chem. Eng., 2015, 3, 3035–3042 CrossRef CAS.
- C. D. Wagner, W. M. Riggs, L. E. Davis and J. F. Moulder, Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corporation, Physical Electronics Division, USA, 1st edn, 1979 Search PubMed.
- T. A. Clarke and E. N. Rizkalla, Chem. Phys. Lett., 1976, 37, 523–526 CrossRef CAS.
- J. Contour, A. Salesse, M. Froment, M. Garreau, J. Thevenin and D. Warin, J. Microsc. Spectrosc. Electron., 1979, 4, 483–491 CAS.
- B. Song, M. O. Lai, Z. Liu, H. Liu and L. Lu, J. Mater. Chem. A, 2013, 1, 9954–9965 CAS.
- F. Cheng, J. Chen, H. Zhou and A. Manthiram, J. Electrochem. Soc., 2013, 160, A1661–A1667 CrossRef CAS.
- N. Yabuuchi, K. Yoshii, S. T. Myung, I. Nakai and S. Komaba, J. Am. Chem. Soc., 2011, 133, 4404–4419 CrossRef CAS PubMed.
- F. Wu, N. Li, Y. Su, H. Shou, L. Bao, W. Yang, L. Zhang, R. An and S. Chen, Adv. Mater., 2013, 25, 3722–3726 CrossRef CAS PubMed.
- Q. B. Xia, X. F. Zhao, M. Q. Xu, Z. P. Ding, J. T. Liu, L. B. Chen, D. G. Ivey and W. F. Wei, J. Mater. Chem. A, 2015, 3, 3995–4003 CAS.
- E. Zhao, X. Liu, H. Zhao, X. Xiao and Z. Hu, Chem. Commun., 2015, 51, 9093–9096 RSC.
- D. Mohanty, J. Li, D. P. Abraham, A. Huq, E. A. Payzant, D. L. Wood and C. Daniel, Chem. Mater., 2014, 26, 6272–6280 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00769d |
| This journal is © The Royal Society of Chemistry 2016 |