Fe3O4/rGO nanocomposite: synthesis and enhanced NOx gas-sensing properties at room temperature†
Ying Yang*ab,
Li Sunc,
Xiangting Dong*a,
Hui Yua,
Tingting Wanga,
Jinxian Wanga,
Ruihong Wangb,
Wensheng Yua and
Guixia Liua
aKey Laboratory of Applied Chemistry and Nanotechnology at Universities of Jilin Province, Changchun University of Science and Technology, Changchun 130022, China. E-mail: yangying0807@126.com; dongxiangting888@163.com; Fax: +86 0431 85383815; Tel: +86 0431 85582574
bKey Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), Ministry of Education, Harbin 150080, P. R. China
cCollege of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar 161006, P. R. China
First published on 29th March 2016
Abstract
We demonstrate a facile fabrication of Fe3O4 nanoparticle (NP)–decorated reduced graphene oxide (Fe3O4/rGO) nanocomposites and their application for the fast and selective detection of NOx at room temperature. The as-synthesized nanocomposites have layered structures and the Fe3O4 NPs with diameters ranging from 30 to 50 nm were evenly loaded on the rGO surface. Furthermore, the Fe3O4/rGO nanocomposite based gas sensor exhibits excellent sensitivity and fast response to NOx gas at room temperature. Moreover, for a NOx concentration of 97.0 ppm, the observed value of sensitivity was about 35.6%, while the response time was 29.3 s. We found that the loading density of the Fe3O4 NPs greatly affects the sensing performance of the Fe3O4/rGO nanocomposites and a suitable NP loading leads to the highest sensitivity. The synthesis method to produce rGO-based nanocomposites as novel gas sensor materials has great potential to push low cost and gas sensing nanotechnology.
Introduction
The design and fabrication of gas sensing devices is considered to be one of the most important technological developments for public security, biological detection and monitoring of agriculture, medical, and manufacturing environments. As typical novel portable gas sensing devices with low power, low cost, fast response and high sensitivity, and chemical micro-gas-sensors have become the leading candidates, which rely on monitoring direct changes in the resistance in adsorption and desorption of the target gas molecules.1–3 Among various gas sensor materials, metal oxides, such as SnO2,4,5 ZnO,6,7 MoO3 (ref. 8,9) and WO3,10,11 have been successfully applied in gas sensing devices. When a metal oxide is exposed to a gas, its resistance varies in accordance with the gas concentration. The theory for the operation of such sensors involves adsorption/desorption phenomena and reactions at the surface of the metal oxide.12 Especially, pure Fe3O4, as an excellent sensing material, has been extensively studied because of its high sensitivity, and good stability.13–15 However, the electrical conductivity of pure Fe3O4 is poor, this fault limits the scope of the efforts towards developing high sensitivity room temperature gas sensors. The introduction of a graphitized carbon substrate with excellent electrical conductivity at room temperature, such as carbon nanotubes (CNTs)16,17 and graphene (GN)18,19 in graphitized carbon-based composites, can compensate for the low electrical conductivity for pure Fe3O4.Graphene has been considered as an excellent candidate for gas sensing applications mainly due to the following two merits: (1) its two-dimensional honeycomb structure, which can easily provide a high surface area, leading to high sensitivity, to the various gas molecules and (2) its inherently low electric noise due to the high quality of its crystal lattice.20,21 However, the sensing performance of the gas sensors based on single graphene is not good enough for practical applications. Based on the abovementioned analysis, to improve the sensing performance of these graphene-based sensors, many sensing materials, such as metals, conducting polymers and metal oxides, have been decorated on the surface of graphene sheets and play an important part in the improvement of the sensitivity and selectivity to the target gas sensors. To the best of our knowledge, there are few reports on room temperature gas sensor based on the Fe3O4/rGO nanomaterials. Therefore, we tried to combine some of the advantages of rGO with Fe3O4 to develop a novel room temperature NOx sensor. The graphene plane would be favorable for the dispersion and stability of Fe3O4 nanoparticles to achieve small sized uniform NPs. Moreover, the graphene plane can act as a substrate to improve the capture and migration of electrons from the conduction band in the nanocomposites.
Herein, we demonstrate a facile fabrication of Fe3O4 NPs–decorated reduced graphene oxide nanocomposites using a solvothermal – pyrolytic method. The gas sensing properties of the resulting Fe3O4/rGO nanocomposites were investigated. The results show that the repeatability and sensitivity of the gas sensor devices for NOx detection can be remarkably enhanced via decorating Fe3O4 NPs on the surface of rGO. The Fe3O4/rGO nanocomposites can offer room temperature NOx gas sensing properties, suggesting their great potential in sensing related applications.
Experimental
Sample preparation
Material characterization
The structures and compositions of the as-prepared products were characterized by XRD (D/max-IIIB-40 kV, Japan, Cu-Kα radiation, λ = 1.5406 Å). The morphologies of the synthesized samples were studied by SEM (Philips XL-30-ESEM-FEG, 5–20 kV) and transmission electron microscopy (TEM, JEOL-JEM-2100, 200 kV). X-ray photoelectron spectroscopy (XPS) analysis was carried out using a VGESCALAB MK II with Mg-Kα (1253.6 eV) achromatic X-ray radiation. The Brunauer–Emmett–Teller (BET) surface area of the products was measured using N2 adsorption–desorption (TriStar II 3020); the sample was dried for 10 h at 150 °C under vacuum before the measurement.Fabrication and measurements of gas sensors
An interdigitated Au electrode (7 × 5 × 0.38 mm) was selected for gas sensing detection and the electrode spacing was 20 μm. A certain amount of sample was dispersed in water to form a suspension. Then, the suspension was spin-coated onto the interdigitated electrode to form a sensitive film and dried at 70 °C for 5 h to obtain a thin film gas sensor. The sensor was installed into a test chamber with an inlet and an outlet. The chamber was flushed with air for 2 min to remove any contaminants from the flask and also to stabilize the film before testing. A syringe was used to inject the required volume of NO vapor into the chamber. The changes in electrical resistance of the sample over time were recorded using a home-made automatic resistance apparatus and the chamber was purged with air to recover the sensor resistance. The sensor sensitivity was defined as the ratio (RN − RA)/RA, where RA is the sensor resistance in air and RN is the resistance in NOx gas. The response time is defined as the time required for the variation in resistance to reach 85% of the equilibrium value after a test gas was injected. The test was conducted at room temperature (20 °C) with a relative humidity (RH) around 40%.Results and discussion
Composition and morphology
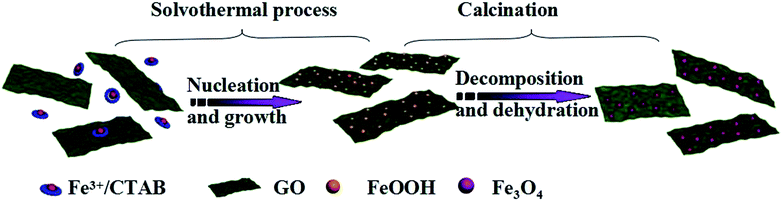
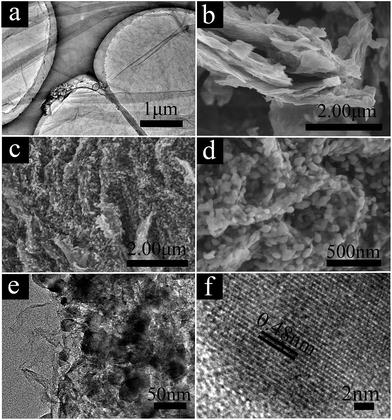
The Fe3O4/rGO nanocomposite was synthesized via a solvothermal-pyrolytic method, as shown in Scheme 1. In the synthesis, Fe3+, CTAB and GO were employed as the Fe source, surfactant and carbon precursor, respectively. Followed by the solvothermal process, Fe3+ was converted into FeOOH NPs reacting with residual oxygen groups of GO. Then, the FeOOH NPs grown on the surface of the GO to obtain the uniform FeOOH/GO nanocomposite. After sintering at 400 °C under a nitrogen atmosphere, the Fe3O4/rGO nanocomposite was prepared.The morphology of the composites was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Fig. 1a shows the TEM image of the obtained GO. The GO sheet appears as a transparent grey film and the wrinkled area could be clearly observed.
For a typical sample (FeOOH/GO2), a low-magnification SEM image, shown in Fig. 1b, reveals the layered nature of the β-FeOOH nanoparticles (NPs) grown on the GO substrate. As observed from SEM (Fig. 1c), Fe3O4/rGO2-400 presents a layered structure and the Fe3O4 NPs were evenly loaded on the rGO surface. The large magnified image (Fig. 1d) indicates the size of Fe3O4 NPs was mainly below 50 nm. The TEM image (Fig. 1e) also revealed the Fe3O4/rGO2-400 with a unique layered nanostructure. From the TEM image (Fig. 1e), we can observe the small particles with good dispersion on a large scale. Fig. 1f shows the HRTEM image of selected regions in Fig. 1e. Fig. 1e indicates that the lattice fringe spacing between the (111) planes (d111) was about 0.48 nm, which is consistent with that of Fe3O4 (JCPDS no. 72-2303; a = 8.4 Å).
In the synthesis, we found that the Fe ion concentration not only influenced the FeOOH/GO nanocomposites morphologies critically, but also decided the morphology and the gas sensing properties of the Fe3O4/rGO nanocomposites. The SEM results indicate that when the mass ratio of the FeCl3·6H2O to the GO was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2a and b) and 10
1 (Fig. 2a and b) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 2c and d), the FeOOH NPs are small and grow uniformly on the surface of nanocomposites. However, extensive aggregation of Fe3O4 NPs occurred, when further increasing the mass of FeCl3·6H2O to 750 mg and 1.0 g for FeOOH/GO3 and FeOOH/GO4, as observed in Fig. 2e–h. The SEM images of the prepared Fe3O4/rGO1-400, Fe3O4/rGO2-400, Fe3O4/rGO3-400 and Fe3O4/rGO4-400 are shown in Fig. 3. In Fig. 3a and b, small amounts of Fe3O4 NPs were loaded on the surface of the rGO and Fe3O4 NPs were overlapped and aggregated together, as shown in Fig. 3d.
1 (Fig. 2c and d), the FeOOH NPs are small and grow uniformly on the surface of nanocomposites. However, extensive aggregation of Fe3O4 NPs occurred, when further increasing the mass of FeCl3·6H2O to 750 mg and 1.0 g for FeOOH/GO3 and FeOOH/GO4, as observed in Fig. 2e–h. The SEM images of the prepared Fe3O4/rGO1-400, Fe3O4/rGO2-400, Fe3O4/rGO3-400 and Fe3O4/rGO4-400 are shown in Fig. 3. In Fig. 3a and b, small amounts of Fe3O4 NPs were loaded on the surface of the rGO and Fe3O4 NPs were overlapped and aggregated together, as shown in Fig. 3d.
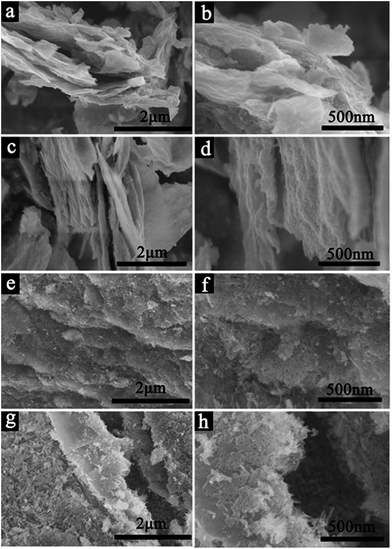 | ||
| Fig. 2 The SEM images of the prepared (a and b) FeOOH/GO1, (c and d) FeOOH/GO2, (e and f) FeOOH/GO3 and (g and h) FeOOH/GO4. | ||
 | ||
| Fig. 3 The SEM images of the prepared (a) Fe3O4/rGO1-400, (b) Fe3O4/rGO2-400, (c) Fe3O4/rGO3-400 and (d) Fe3O4/rGO4-400. | ||
The aggregation of the NPs will affect the gas sensing properties of the graphene-like nanosheet composites, which has been confirmed in our previous work.
The wide-angle powder XRD patterns of raw FeOOH/GO are displayed in Fig. 4. In Fig. 4a, the broad diffraction peak at 2θ = 12.2° can be indexed to the (001) plane of graphite oxide. The XRD pattern of the as-prepared FeOOH/GO nanoparticles conformed to the cubic structure of β-FeOOH (PDF 75-1594) and no characteristic peaks were observed for other impurities in Fig. 4b–e. The missing diffraction peaks of the (001) planes (GO) and (002) planes (G) may be due both to the partial reduction of the GO and to the coverage effect of the strong diffraction peaks of β-FeOOH. The peak at 12.2° in curve a is characteristic for GO with an interlayer spacing of 0.85 nm, resulting from facile exfoliation because of weakened the van der Waals forces between the layers of GO.
The as-prepared FeOOH powders after calcination at 300 and 400 °C were identified as a crystalline Fe3O4 phase (JCPDS 76-1849) (Fig. 5a and b). When heat treated at 500 and 600 °C for 2 h, the powders were transformed into Fe2O3 with minor Fe3O4 (Fig. 5c and d). The XRD pattern of γ-Fe2O3 (JCPDS 39-1346) was also similar to that of Fe3O4. In the TEM analysis, the lattice fringe spacing between the (111) planes (d111) was about 0.48 nm, which is consistent with that of Fe3O4. This indicates that the as-prepared sample in Fig. 5a can be identified as Fe3O4 rather than γ-Fe2O3. It was found that the calcination conditions have remarkable effects on the structure and the sensing properties. The optimum calcination temperature for the Fe3O4 powders prepared was about 400 °C.
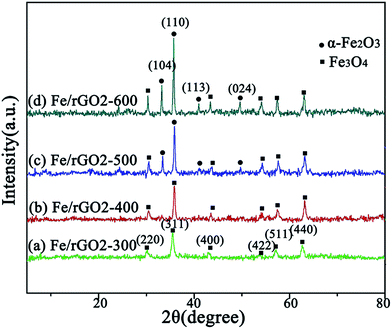 | ||
| Fig. 5 XRD patterns of (a) Fe3O4/rGO2-300, (b) Fe3O4/rGO2-400, (c) Fe3O4/rGO2-500 and (d) Fe3O4/rGO2-600. | ||
To study the influence of the chemical composition of the Fe3O4/rGO nanocomposite, valence for the materials and the interaction between different components, X-ray photoelectron spectroscopy (XPS) was carried out. The reduction of GO in the Fe3O4/rGO nanocomposites was further characterized by XPS to detect the different chemical states of GO before and after calcination. Fig. 6 shows the C 1s XPS spectra of GO (Fig. 6a) and reduced GO in the Fe3O4/rGO nanocomposite (Fig. 6b–d). The XPS spectrum of C 1s can be deconvoluted into four peaks centered at 284.9, 285.7, 287.4 eV and 289.3 eV. The peak at 284.6 eV was attributed to the sp2 carbon atoms (C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–H), whereas the peak positioned at 285.7 and 287.4 eV was assigned to the C from the C–OH and C
C, and C–H), whereas the peak positioned at 285.7 and 287.4 eV was assigned to the C from the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. The peak located at 289.3 eV was closely associated with the O
O groups. The peak located at 289.3 eV was closely associated with the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH species.23,24 However, the C–OH and C
C–OH species.23,24 However, the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal decreased markedly in intensity, suggesting the effective elimination of the oxygen-containing groups in graphene oxide. Therefore, it can be concluded that the GO in the Fe3O4/rGO nanocomposites was almost completely reduced to graphene. The O 1s spectrum is shown in Fig. S1.† The observed peak was asymmetric with a shoulder on the higher binding energy side, indicating more phases of the O element. There maybe three phases of the O element contributing to the O 1s spectrum: the O element in the Fe3O4 NCs, the O element in the organic ligand and contamination oxygen from air.
O signal decreased markedly in intensity, suggesting the effective elimination of the oxygen-containing groups in graphene oxide. Therefore, it can be concluded that the GO in the Fe3O4/rGO nanocomposites was almost completely reduced to graphene. The O 1s spectrum is shown in Fig. S1.† The observed peak was asymmetric with a shoulder on the higher binding energy side, indicating more phases of the O element. There maybe three phases of the O element contributing to the O 1s spectrum: the O element in the Fe3O4 NCs, the O element in the organic ligand and contamination oxygen from air.
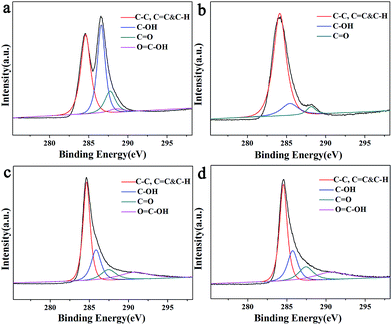 | ||
| Fig. 6 The XPS spectra of C 1s (a) GO, (b) Fe3O4/rGO1-400, (c) Fe3O4/GO2-400 and (d) Fe3O4/rGO3-400. | ||
The formation of the porous structure of the Fe3O4/rGO nanocomposites was verified by nitrogen adsorption/desorption measurements (Fig. 7) and the corresponding pore size distribution was given, as the inset of Fig. 7. All the Fe3O4/rGO nanocomposites show typical type IV curves with clear H3-type hysteresis loops in the relative pressure region of 0.4–0.8 P/P0 and slightly steep adsorption a at low relative pressure, which indicates the presence of slit-like mesopores (2–50 nm) in all of the samples.25,26 In Fig. 7a, the BET surface area of the Fe3O4 NPs was determined to be 57.13 m2 g−1. The inserted figure is the pore size distribution of the Fe3O4 NPs and the average pore width of the sample was about 23.01 nm. When compared with the Fe3O4 NPs, the Fe3O4/rGO-400 nanocomposite exhibits relatively higher specific surface areas of 79.61, 160.39 and 172.03 m2 g−1. Their average pore widths are 14.29, 8.87 and 7.46 nm. Considering the morphologies of the Fe3O4/rGO-400 nanocomposite, the increase in the BET-surface areas of the layered structures was mainly attributed to the increase in GO. The results of the surface area, pore volume and pore size for Fe3O4/rGO1-400, Fe3O4/rGO2-400, Fe3O4/rGO3-400 and Fe3O4 are shown in Table S1.†
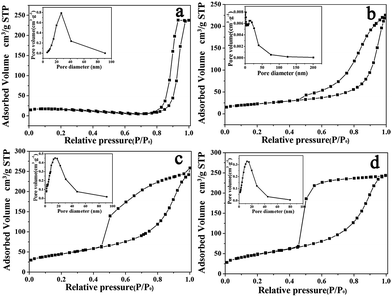 | ||
| Fig. 7 Nitrogen adsorption–desorption isotherms and pore diameter distribution of (a) Fe3O4, (b) Fe3O4/rGO1-400, (c) Fe3O4/rGO2-400, and (d) Fe3O4/rGO3-400. | ||
Gas-sensing performance
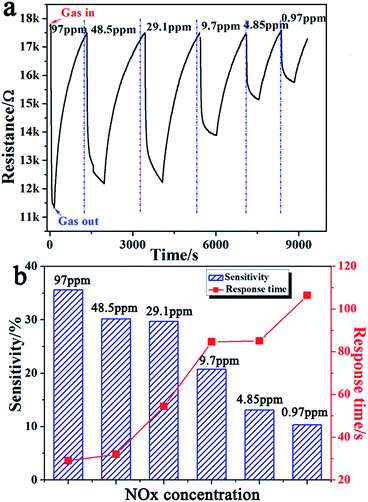
It is known that the sensitivity of the gas sensors is mainly related to the gas concentration. To study the gas sensor properties of the Fe3O4/rGO nanocomposites, a series of experiments were carried out by varying the NOx gas concentration from 97.0 to 0.97 ppm. All these tests were made at room temperature (25 °C, humidity 26%). Fig. 8 shows the dynamic response and recovery curves of the Fe3O4/rGO2-400 sensor as a NOx gas sensor at RT. The sensitivity noticeably increased with an increase in the gas concentration. Taking the Fe3O4/rGO2-400 sensor as an example, the sensitivity decreased from 35.6 to 20.7 and 10.3% when the concentration was decreased from 97.0 ppm to 9.7 ppm and 0.97 ppm (Fig. 8a and Table S2†). From the abovementioned measured results, the sensors have a wide detection range from 0.97 to 97.0 ppm for NOx gas. The corresponding relationship between the sensor response and response time under different NOx concentrations is shown in Fig. 8b and Table S3.† In Fig. 9, a few cycles were recorded for the samples of Fe3O4/rGO1-400, Fe3O4/rGO2-400, Fe3O4/rGO3-400, Fe3O4/rGO4-400 and Fe3O4, at a concentration of NOx gas ranging from 97.0 ppm to 0.97 ppm. The dynamic response and recovery curves of the Fe3O4/rGO1-400, Fe3O4/rGO3-400, Fe3O4/rGO4-400 and Fe3O4 sensors as NOx gas sensors at RT are shown in Fig S2.† With the concentration of NOx decreasing, the sensitivity will decrease and response time will be lengthened for the four samples. Especially for the Fe3O4/rGO2-400 sensor, the sensitivity increased almost 7.3 fold as for pristine ones, which indicated that the novel nanostructure and synergistic effect play essential roles for enhancing the response of gas sensors. To prevent possible loss and disasters, not only the gas response, but also the response time should be enhanced. In particular, the latter is required for real-time monitoring of toxic and hazardous gases.27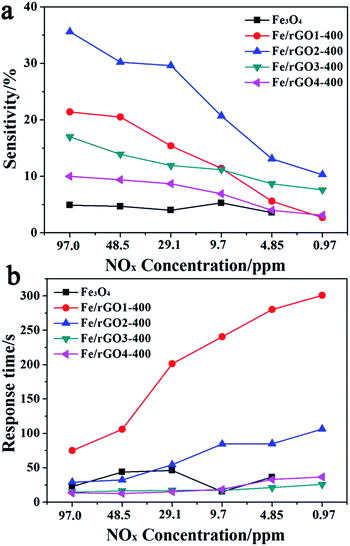 | ||
| Fig. 9 The linear graphs of (a) the sensitivity and (b) response time for the Fe/rGO1-400, Fe/rGO2-400, Fe/rGO3-400, Fe/rGO4-400 and Fe3O4 sensors to 97.0–0.97 ppm NOx operated at room temperature. | ||
In view of practical and commercial applications, the sensor selectivity is an important factor in gas sensing.28,29 Considering the selectivity of the as-prepared Fe3O4/rGO2-400 sensor, we have detected O2, CO, C2H2, H2 and NH3 gas with the same concentration level at room temperature and the results are shown in Fig. S3.† From the gas response bar graph, we find that the measured responses to 100 ppm NOx (35.6%) were higher than the corresponding values for 100 ppm NH3, but it is totally insensitive to O2, H2, CO, and C2H2 at the same concentration of 100 ppm. The sensor exhibits a higher gas response to NOx than to other gases, which is mainly because of the enhanced reaction between the NOx and the absorbed oxygen (O2−, O−) at room temperature.30,31
Moreover, we note that the gas response of the Fe3O4/rGO2-400 sensor presents a favorable linearity (y = 12.83x + 8.48) and correlation coefficient (R2 = 0.97139) in the investigated concentration range from 0.97 to 1000 ppm by plotting the gas response against log[NOx] (Fig. S4†). From the calibration curves it is possible to extract information about the gas response and an unknown concentration of NOx gas can then be identified by comparing its response value to the reference curve. Thus, the Fe3O4/rGO2-400 sensor is suitable for commercial application to NOx sensing detection.
The sensing mechanism and the controllability in sensitivity of the sensor were suggested as follows. When the P-type Fe3O4 semiconductor was used in the gas sensor and exposed to air, O2 molecules will be chemisorbed and capture some electrons of Fe3O4 to be changed into O2−, O−, and O2− on the sensing body surface.32 The oxygen species can then establish a chemical equilibrium:
| O2 ↔ O2− ↔ O− ↔ O2− |
Such chemisorbed oxygen is suggested to act as an electron donor, which depends strongly on the temperature and the nature of the material. The formation of numerous oxygen species (e.g. O2−, O−, and O2−), which are known for their good catalytic activity in gas sensors, can improve the performance of the NOx sensor.33,34 After the oxidizing gas (e.g., NOx) was introduced, some oxygen species will be reduced and removed from the surface above a certain temperature, resulting in the variation of the resistance of Fe3O4. The surface area and the morphology of a sensing film can directly decide the adsorption quantity of the oxygen species and therefore have important impacts on the variation of resistance and the relevant sensitivity of the sensors. Schematic for sensing mechanism is shown in Fig. 10. When the sensor film was exposed to NOx, the NOx gas molecules can attract the electrons from the Fe3O4/rGO nanocomposite sensor because of the high electron affinity of the NOx molecules, which leads to electron transfer from the Fe3O4/rGO nanocomposite.
 | ||
| Fig. 10 (a and b) SEM images of the Fe3O4/rGO nanocomposite, (c) schematic for mechanism of NOx sensing of the Fe3O4/rGO nanocomposite sensor. | ||
From all the abovementioned findings, the significantly improved gas sensing for NOx of our Fe3O4/rGO nanocomposite sensor can be ascribed to the following two factors. First, this enhanced gas sensing is believed to be related to their improved conductivity as described above. As we know, for a resistance-type sensor, the principle of gas detection is based on the conductance variation of the sensing element, which depends on the gas atmosphere and on the operating temperature of the sensing material exposed to the tested gas. It has been reported that graphene can adsorb gas molecules and that the adsorbed molecules change the local carrier concentration in graphene by one electron. Therefore, the improved carrier mobility in graphene of the Fe3O4/rGO-400 with low noise sensing was responsible for this enhanced gas sensing. In addition, rGO as the substrate not only enhanced the conductivity of the sensor component, but also created a Schottky contact at the interface with the semi-conductive oxide.35,36 Second, effective gas diffusion from slit pores between the parallel layers of graphene may also contribute to this gas sensing enhancement. The slit pores can act as channels for gas diffusion and thus provide more active sites for the reaction of alcohol with surface-adsorbed oxygen ions. Although the existed pores for the as-synthesized Fe3O4/rGO-400 also provide channels for the tested gas to diffusion, the tested gas can react with functional groups and defects from GO in Fe3O4/rGO-400, leading to the hampering of gas diffusion to the sensing surface.37
Conclusions
We have demonstrated that Fe3O4/rGO nanocomposites can be prepared via a solvent-thermal method. The as-synthesized nanocomposites have layered structures and the Fe3O4 NPs with diameters about 50 nm were evenly loaded on the rGO surface. When compared with the pure Fe3O4, the Fe3O4/rGO nanocomposites based gas sensor exhibits more excellent sensitivity and faster response to NOx gas at room temperature. This enhanced gas sensing is believed to be ascribed to the following two factors. The first is believed to be related to the improved conductivity in the nanocomposites. The other is attributed to the effective gas diffusion from slit pores between the parallel layers of rGO.Acknowledgements
We gratefully acknowledge the support of this study by the National Natural Science Foundation of China (No. 51572034, 21501104), the Natural Science Foundation of Heilongjiang Province (B2015014), and the Natural Science Foundation of Changchun University of Science and Technology (No. XQNJJ-2015-07).Notes and references
- D. J. Wales, J. Grand, V. P. Ting, R. D. Burke, K. J. Edler, C. R. Bowen, S. Mintova and A. D. Burrows, Chem. Soc. Rev., 2015, 44, 4290–4321 RSC.
- D. C. Pugh, V. Luthra, A. Singh and I. P. Parkin, RSC Adv., 2015, 5, 85767–85774 RSC.
- Y. S. Liu, P. Yang, J. Li, K. M. Postolek, Y. L. Yue and B. C. Huang, RSC Adv., 2015, 5, 98500–98507 RSC.
- X. Liu, N. Chen, B. Q. Han, X. C. Xiao, G. Chen, I. Djerdj and Y. D. Wang, Nanoscale, 2015, 7, 14872–14880 RSC.
- H. H. Yan, P. Song, S. Zhang, Z. X. Yang and Q. Wang, RSC Adv., 2015, 5, 79593 RSC.
- X. M. Wang, F. Z. Sun, Y. Q. Duan, Z. P. Yin, W. Luo, Y. A. Huang and J. K. Chen, J. Mater. Chem. C, 2015, 3, 11397–11405 RSC.
- S. Zhang, H. S. Chen, K. Matras-Postolek and P. Yang, Phys. Chem. Chem. Phys., 2015, 17, 30300 RSC.
- X. M. Gao, C. Y. Li, Z. X. Yin and Y. J. Chen, RSC Adv., 2015, 5, 37703 RSC.
- N. Illyaskutty, S. Sreedhar, G. S. Kumar, H. Kohler, M. Schwotzer, C. Natzeck and V. P. M. Pillai, Nanoscale, 2014, 6, 13882 RSC.
- Z. H. Li, J. C. Li, L. L. Song, H. Q. Gong and Q. Ni, J. Mater. Chem. A, 2013, 1, 15377–15382 CAS.
- Z. Y. Wang, X. Zhou, Z. B. Li, Y. C. Zhuo, Y. Gao, Q. Y. Yang, X. W. Li and G. Y. Lu, RSC Adv., 2014, 4, 23281 RSC.
- X. L. Li, T. J. Lou, X. M. Sun and Y. D. Li, Inorg. Chem., 2004, 43, 5442 CrossRef CAS PubMed.
- Z. H. Ai, K. J. Deng, Q. F. Wan, L. Z. Zhang and S. C. Lee, J. Phys. Chem. C, 2010, 114, 6237–6242 CAS.
- S. O. Hwang, C. H. Kim, Y. Myung, S. H. Park, J. Park, J. Kim, C.-S. Han and J.-Y. Kim, J. Phys. Chem. C, 2008, 112, 13911–13916 CAS.
- X. Liu, J. W. Li, J. B. Sun and X. T. Zhang, RSC Adv., 2015, 5, 73699–73704 RSC.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, Chem. Soc. Rev., 2013, 42, 2824 RSC.
- Y. Lin, D. W. Baggett, J. W. Kim, E. J. Siochi and J. W. Connell, ACS Appl. Mater. Interfaces, 2011, 3, 1652 CAS.
- B. H. Chu, C. F. Lo, J. Nicolosi, C. Y. Chang, V. Chen, W. Strupinski, S. J. Pearton and F. Ren, Sens. Actuators, B, 2011, 157, 500 CrossRef CAS.
- W. W. Li, X. M. Geng, Y. F. Guo, J. Z Rong, Y. P. Gong, L. Q. Wu, X. M. Zhang, P. Li, J. B. Xu, G. S. Cheng, M. T. Sun and L. W. Liu, ACS Nano, 2011, 5, 6955 CrossRef CAS PubMed.
- Z. X. Jiang, J. Li, H. Aslan, Q. Li, Y. Li, M. L. Chen, Y. D. Huang, J. P. Froning, M. Otyepka, R. Zbořil, F. Besenbacher and M. Dong, J. Mater. Chem. A, 2014, 2, 6714–6717 CAS.
- B. Cho, J. Yoon, M. Gwan Hahm, D.-H. Kim, A. R. Kim, Y. H. Kahng, S.-W. Park, Y.-J. Lee, S.-G. Park, J.-D. Kwon, C. S. Kim, M. Song, Y. Jeong, K.-S. Nam and H. C. Ko, J. Mater. Chem. C, 2014, 2, 5280–5285 RSC.
- V. C. Tung, L. M. Chen, M. J. Allen, J. K. Wassei, K. Nelson, R. B. Kaner and Y. Yang, Nano Lett., 2009, 9, 1949–1955 CrossRef CAS PubMed.
- O. Akhavan, ACS Nano, 2010, 4, 4174 CrossRef CAS PubMed.
- Z. B. Lei, L. Lu and X. S. Zhao, Energy Environ. Sci., 2012, 5, 6391 CAS.
- Z. Xie, Y. G. Zhu, J. Xu, H. T. Huang, D. Chen and G. Z. Shen, CrystEngComm, 2011, 13, 6393 RSC.
- R. Pierotti and J. Rouquerol, Pure Appl. Chem., 1985, 57, 603 Search PubMed.
- Z. Lou, F. Li, J. N. Deng, L. L. Wang and T. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 12310 CAS.
- C. W. Na, H. S. Woo, I. D. Kim and J. H. Lee, Chem. Commun., 2011, 47, 5148 RSC.
- D. Barreca, G. Carraro, E. Comini, A. Gasparotto, C. Maccato, C. Sada, G. Sberveglieri and E. Tondello, J. Phys. Chem. C, 2011, 115, 10510 CAS.
- D. H. Zhang, Z. Q. Liu, C. Li, T. Tang, X. L. Liu, S. Han, B. Lei and C. W. Zhou, Nano Lett., 2004, 4, 1919 CrossRef CAS.
- R. J. Lv, K. Y. Shi, W. Zhou, L. Wang, C. G. Tian, K. Pan, L. Sun and H. G. Fu, J. Mater. Chem., 2012, 22, 24814 RSC.
- X. J. Huang, F. L. Meng, Z. X. Pi, W. H. Xu and J. H. Liu, Sens. Actuators, B, 2004, 99, 444 CrossRef CAS.
- R. J. Lv, K. Y. Shi, W. Zhou, L. Wang, C. G. Tian, K. Pan, L. Sun and H. G. Fu, J. Mater. Chem., 2012, 22, 24814 RSC.
- Y. Yang, C. G. Tian, J. C. Wang, L. Sun, K. Y. Shi, Z. Wei and H. G. Fu, Nanoscale, 2014, 6, 7369–7378 RSC.
- S. Z. Deng, V. Tjoa, H. M. Fan, H. R. Tan, D. C. Sayle, M. Olivo, S. Mhaisalkar, J. Wei and C. H. Sow, J. Am. Chem. Soc., 2012, 134, 4905 CrossRef CAS PubMed.
- Z. Y. Zhang, R. J. Zou, G. S. Song, L. Yu, Z. G. Chen and J. Q. Hu, J. Mater. Chem., 2011, 21, 17360 RSC.
- K. R. Ratinac, W. R. Yang, S. P. Ringer and F. Braet, Environ. Sci. Technol., 2010, 44, 1167 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02306a |
| This journal is © The Royal Society of Chemistry 2016 |