Synthesis of peptide nanofibers decorated with palladium nanoparticles and its application as an efficient catalyst for the synthesis of sulfides via reaction of aryl halides with thiourea or 2-mercaptobenzothiazole
Abstract
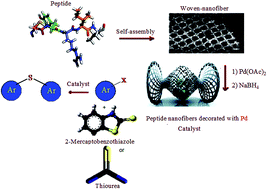
In this work supported Pd nanoparticles on a peptide nanofiber (PdNP–PNF) have been prepared via fabrication of self-assembled woven nanofiber from peptide, subsequently immobilization of palladium nanoparticles on this nanostructural compound. To obtain self-assembled woven nanofiber, we designed and synthesized a peptide using arginine as building block. The C-terminus of amino acid was protected as ethylester. Coupling was mediated by dicyclohexylecarbodiimide-1-hydroxybenzotriazole (DCC-HOBT). TEM, SEM, XRD, ICP and FT-IR techniques were employed to characterize prepared nanofiber materials. In this work, the effect of phosphate buffer solutions pH 8 and pH 11 (isoelectric point of arginine amino acid) on the structure of peptide nanofiber was investigated. Supported Pd nanoparticles on the peptide nanofiber (PdNP–PNF) were applied for the C–S coupling reaction using two different sulfur transfer reagents (thiourea and 2-mercaptobenzothiazole).

 Please wait while we load your content...
Please wait while we load your content...