Green synthesis of palladium nanoparticles via branched polymers: a bio-based nanocomposite for C–C coupling reactions†
Abstract
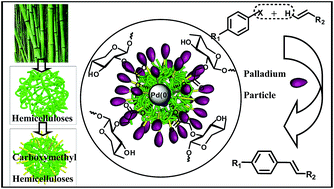
Catalytic process is the key process for many chemical industries. In this study, a novel heterogeneous Pd (CMH–Pd(0)) has been prepared by the deposition of palladium nanoparticles (Pd NPs) onto the surface of carboxymethyl functionalized hemicelluloses using ethanol as solvent and in situ reducing agent. The as prepared catalyst was characterized by TEM, HR-TEM, XRD, FT-IR, TGA and XPS. The loading level of Pd in the CMH–Pd(0) catalyst was 0.38 mmol g−1. The catalyst showed high catalytic activity and versatility towards Heck coupling reactions under aerobic conditions and could be readily recovered and reused in at least five successive cycles without obvious loss in activity. The catalyst is promising for its renewability, environmental benefits, efficient catalytic activity, mild reaction conditions, simple product work-up and easy catalyst recovery.

 Please wait while we load your content...
Please wait while we load your content...