Fabrication of laminated and coated Nafion 117 membranes for reduced mass transfer in microbial fuel cells†
Abstract
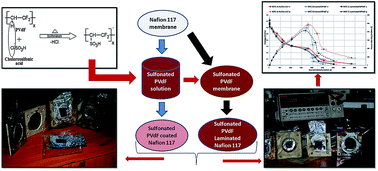
Sulfonation of polyvinylidinefluoride (PVdF) with chlorosulfonic acid for 2 hours revealed a respective 33% degree of sulfonation (DS) in a SPVdF membrane. The resulting sulfonated PVdF resins were used for Nafion 117 modification as coat and laminating materials, where the modified Nafion 117 membranes (laminated and coated with SPVdF) were used as polymer electrolyte membranes in microbial fuel cells (MFCs). Coating/lamination exhibited reduced oxygen diffusion across membranes by a magnitude of less than two orders over a pristine Nafion 117 membrane. This resulted in higher open circuit voltages (OCVs) with increased coulombic efficiency (CE) in MFCs. With SPVdF coated and laminated Nafion 117 membranes, respective IEC values of 0.57 and 0.46 meq g−1 and proton conductivities of 5.91 × 10−3 and 5.11 × 10−3 S cm−1 were observed, indicating maximum power and current densities of 446.45 ± 21 mW m−2 & 1721.78 ± 86 mA m−2 and 413.79 ± 20 mW m−2 & 1657.57 ± 82 mA m−2 in MFCs using mixed Firmicutes as biocatalysts. The obtained coulombic efficiencies were higher (approx. 1–2%) than those of pristine Nafion 117, indicative of its reduced oxygen diffusion at the anode. In effect, the study enumerates the efficiency of modified Nafion 117 with sulfonated PVdF coated and laminated membranes as a separating barrier in single-chambered MFCs for microbial bio-energy conversion.

 Please wait while we load your content...
Please wait while we load your content...