Enhanced corrosion resistance of polybenzoxazine coatings by epoxy incorporation
Changlu Zhoua,
Jiaping Linb,
Xin Luc and
Zhong Xin*c
aShanghai Key Laboratory of Multiphase Materials Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
bSchool of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China
cState Key Laboratory of Chemistry Engineering, School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: xzh@ecust.edu.cn; Fax: +86 21 64251772; Tel: +86 21 64240862
First published on 1st March 2016
Abstract
Polybenzoxazine/epoxy composite coating has been developed on mild steel (MS) substrates by a thermal curing method. The reaction between two components was characterized by Fourier transform infrared spectroscopy (FTIR). The effects of incorporating epoxy on the corrosion resistance of coated steel were investigated by open circuit potential, potentiodynamic polarization, and electrochemical impedance spectroscopy, respectively. The resulted composite coated samples exhibit significant enhancement on the corrosion resistance of MS with the corrosion current reduced by more than one order of magnitude, and the enhancement is improved with an increase in the epoxy content, which benefits from the dual crosslinking network of the composite coating through the incorporation of epoxy. In addition to enhancing the coating barrier performance, all coated samples are substantially hydrophobic with a contact angle of above 100° before and even after the polarization measurements.
Introduction
The past several decades have witnessed active research on polybenzoxazines as advanced thermoset polymers, with significant efforts in tailoring their properties for the specific requirements of individual applications.1–3 Hitherto, some benzoxazine resins have been commercialized by several companies, including Huntsman, Shikoku, and Henkel.4 The outstanding properties of polybenzoxazines make this new class of polymers as alternatives to many traditional polymers (phenolics, epoxies) with attractive performance in the electronics and aerospace industries. As a result of this strong interest and great achievement in polybenzoxazines, researchers on polybenzoxazines are keen to develop this kind of high-performance resin into other industries considering their advantages.5–7Recently, polybenzoxazines have gained increasing attention in corrosion protection area due to their highly attractive features, such as low water absorption, good hydrophobicity, and good dielectric properties. It is recognized that polybenzoxazines act as a stable barrier which can isolate the metal from the corrosive environment and prevent ionic transport and electrical conduction of the corrosion reaction. Besides commercial bisphenol A benzoxazine (10 μm thick) showed excellent corrosion resistance with impedance modulus > 109 Ω cm2 after 30 days immersion in 3.5 wt% NaCl solution,8 and many benzoxazines with novel structures were designed by considering various requirements of corrosion protection, which also exhibit good performance. For instance, silane functionality in benzoxazines can effectively enhance the coatings’ substrate adhesion and the monomers based on nature resources like vegetable oil can make the polybenzoxazine coating environmentally friendly.9–11 As far as these preceding studies are concerned, polybenzoxazines exhibit high potentialities as a promising alternative in corrosion protection for metals.
In general, the attractive characteristics of each component are always apparent when combined as composites, and modified polybenzoxazine coatings by introducing other functional materials can also lead to a significant improvement in processability, thermal stability, mechanical properties, and so forth.12 For corrosion protection, further enhancement and some functionality favourable to anticorrosion can also be obtained when polybenzoxazines combine with other constituents.13,14 More importantly, this is a fascinating path which can promote polybenzoxazine to industry soon. Among various high performance materials to composite, epoxy is known to be the most conventional and good coating material in many aspects due to their excellent chemical resistance, good adhesion properties, etc.15,16 In particular, a stable chemical bond can form between benzoxazine and epoxy molecules because the epoxide group can react with the phenolic hydroxyl groups of polybenzoxazine.17,18 It is believed that the incorporation of an epoxy group could increase the crosslinking density of the composite with benzoxazine, which would play a profound role in the coatings’ barrier ability against corrosion medium during corrosion protection service life.19 Hence, understanding the effect of epoxy on the corrosion resistance of the polybenzoxazine/epoxy coating will be of benefit to the future development of commercial polybenzoxazine-based coatings in addressing the global metallic corrosion challenge.
Herein, our work examines the influence of epoxy incorporation on the anticorrosion behaviour of polybenzoxazine coatings, which were studied through electrochemical techniques and contact angle measurements. The reaction between polybenzoxazine and an epoxide was investigated by Fourier transform infrared (FTIR) spectroscopy. It can provide details of the corrosion mechanism of this composite coating system and thus provide guidance for the design of high-performance polybenzoxazine-based coatings used for corrosion protection of steel.
Results and discussion
Crosslinking reaction between polybenzoxazine and epoxy
Many reports verified that the phenolic hydroxyl group produced by the ring opening reaction of benzoxazine can react with the epoxide group easily under thermal treatment.22–26 In this work, a qualitative infrared analysis was undertaken to investigate possible reaction between BA-aptms and epoxy in the composite system.Fig. 1 illustrates the FTIR spectra of BA-aptms monomer, epoxy precursor and different polymers after heating at 230 °C for 2 h. The typical out-of-plane bending vibrations of C–H of the oxazine ring in benzoxazine is well reflected at 931 cm−1 (Fig. 1b) on the plot of BA-aptms.21 In the case of the epoxy precursor, a peak at 916 cm−1 (Fig. 1a) and a shoulder band at 863 cm−1 (Fig. 1a) can be observed, which can be assigned to the C–O deformation band and stretching C–O–C band of oxirane group of epoxy, respectively.23,27 In contrast, the above characteristic peaks completely disappear by the end of the thermal treatment in all polymer systems and a broad band appears at about 3400 cm−1, which can be attributed to the hydroxyl groups of polybenzoxazine. The peak corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the aromatic rings of the epoxide at 1608 cm−1 (Fig. 1d and e) is clearly obtained on the FTIR curves of PBE composites. Since the epoxide cannot cure itself without a curing agent, the ring opening reaction of the epoxy group should be catalysed by polybenzoxazine in this composite system. Therefore, the results of FTIR spectroscopy point out the occurrence of the ring opening reaction of benzoxazine and etherification hydroxyl-oxazine reaction between BA-aptms and epoxy, which were proved by Kimura and his co-workers through model reaction.23 Due to the originally unoccupied phenolic hydroxyl groups of polybenzoxazine act as the linking bridge between polybenzoxazine and epoxide, dual crosslinking network forms in the composite thermosetting matrix. Therefore, a theoretically possibly denser network could form by this kind of additional crosslinking points’ incorporation to the PBE system, which makes it more effective for the coatings to act as barrier for the penetration of a corrosive medium.
C stretching of the aromatic rings of the epoxide at 1608 cm−1 (Fig. 1d and e) is clearly obtained on the FTIR curves of PBE composites. Since the epoxide cannot cure itself without a curing agent, the ring opening reaction of the epoxy group should be catalysed by polybenzoxazine in this composite system. Therefore, the results of FTIR spectroscopy point out the occurrence of the ring opening reaction of benzoxazine and etherification hydroxyl-oxazine reaction between BA-aptms and epoxy, which were proved by Kimura and his co-workers through model reaction.23 Due to the originally unoccupied phenolic hydroxyl groups of polybenzoxazine act as the linking bridge between polybenzoxazine and epoxide, dual crosslinking network forms in the composite thermosetting matrix. Therefore, a theoretically possibly denser network could form by this kind of additional crosslinking points’ incorporation to the PBE system, which makes it more effective for the coatings to act as barrier for the penetration of a corrosive medium.
 | ||
| Fig. 1 FTIR spectra of epoxy precursor (a), BA-aptms monomer (b), poly(BA-aptms) (c) and PBE composites (d: PBE20, e: PBE40). | ||
Barrier properties of the composite system
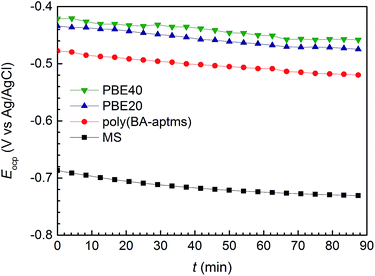
The plots of the open-circuit potentials (Eocp, vs. Ag/AgCl) as a function of the immersion time (t) in the corrosive medium were recorded for the bare and coated samples in Fig. 2. The bare MS shows an initial Eocp of −0.69 V (Ag/AgCl), which decreases to −0.73 V (Ag/AgCl) after ∼90 min immersion. Meanwhile, a remarkable shift of Eocp to the anodic direction can be observed for the coated samples. The initial Eocp and the Eocp after ∼90 min immersion test of the poly(BA-aptms) sample are −0.51 V (Ag/AgCl) and −0.52 V (Ag/AgCl), respectively, and the Eocp values continue to increase with increasing the epoxy content in the PBE system. Since organic coatings are not impenetrable, micro pores in the film would provide a path of water molecules passing through the coatings.28 Therefore, coatings with fewer micro pores prepared from higher density polymer could inhibit the essential step in metal corrosion. In fact, high Eocp typically indicates that the coating are less porous in nature with a lower permeability against diffusing corrosive species by the increased ability to act as barrier.29,30 Considering Fig. 2, it is evident that a sample with high epoxy content not only shows better stability during the immersion time reflected by less reduction of Eocp compared with neat poly(BA-aptms) but it also presents higher Eocp values among the investigated samples. It is possible to note that epoxy incorporation can significantly decrease the micro pores inside the coating matrix through enhancing the crosslinking density of the composite polymer network. It is suggested that effect of epoxy groups on the corrosion protection enhancement of polybenzoxazine coatings is to improve their barrier ability against diffusing corrosive species.Corrosion resistance of the coatings
Fig. 3 shows the polarization curves of MS uncoated or coated with different coatings. Both anodic and cathodic corrosive processes are suppressed evidently to the point that the corrosion current densities obtained from anodic and cathodic branches of Tafel representation decrease appreciably, at least, one decade (from 10−5 A cm−2 to 10−6 A cm−2) for the coated samples compared with those without the coatings. It is evident that the polybenzoxazine coating can inhibit both the cathodic and anodic reactions on the MS surface, and the incorporation of epoxy groups further promotes more than one decade reduction (from 10−6 A cm−2 to 10−7 A cm−2) of the corrosion current compared to the epoxy-free poly(BA-aptms) coated sample. Also, the corrosion potential of PBE samples increases positively with increasing epoxy content from about −0.49 V (Ag/AgCl) to less than −0.45 V (Ag/AgCl), which are much higher than the bare MS with corrosion potential of −0.7 V (Ag/AgCl). All these results as described above prove that the presence of epoxy groups improves the corrosion resistance of the poly(BA-aptms) coating, and the contribution enhances with an increase in epoxy content.Electrochemical behaviours of the PBE coatings
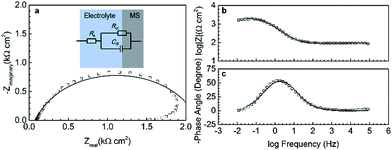
The corrosion resistance of bare MS and the poly(BA-aptms) coated MS, unincorporated or incorporated with different content of epoxy, were further examined by EIS technique at room temperature to better understand the electrochemical behaviours of all investigated samples. The recorded EIS spectra for the bare mild steel in 3.5 wt% NaCl aqueous solutions are depicted in Fig. 4. The shape of Nyquist plot (Fig. 4a) features two time constants: one depressed capacitive semicircle at the higher frequency range, attributed to charge transfer in the corrosion process and another inductive loop that is observed in the lower frequency region is related to the adsorption of an intermediate product in the corrosion reaction.31,32 The EIS data are fitted using only one time constant described by a simple Randles equivalent electrical circuit (EEC) depicted in upper part of Fig. 4a, which includes the three elements: Rs is a resistor related to the solution resistance, Rct (charge transfer resistance) is a measure of electron transfer across the surface and is inversely proportional to corrosion rate,33 and Cdl is the capacitance of the electrical double layer. In terms of Cdl, it is stimulated by the constant phase element (CPE) in the model instead of the ideal electrical capacitance to fit more accurately impedance behaviour of the electric double layer. It can be explained by the parameters n and Y, which are respectively the exponential coefficient and the frequency-independent constant in the CPE’s expression (impedance: ZCPE = 1/Y(jω)n with j is an imaginary unit, ω is an angular frequency (ω = 2πf)). The parameters of the equivalent model are listed in Table 1.| EEC parameters | Bare MS | Poly(BA-aptms) | PBE20 | PBE40 |
|---|---|---|---|---|
| Rs (Ω cm2) | 94.2 ± 4.3 | 75.1 ± 25.3 | 135.5 ± 37.1 | 68.9 ± 12.6 |
| Rct (kΩ cm2) | 2.00 ± 0.12 | 8.65 ± 3.39 | 317.3 ± 15.7 | 941.4 ± 5.9 |
| Cdl: Y (Ω−1 cm−2 sn) | 3.08 ± 0.28 × 10−4 | 2.85 ± 0.66 × 10−7 | 4.15 ± 0.20 × 10−8 | 9.41 ± 5.71 × 10−9 |
| Cdl: n | 0.83 ± 0.01 | 0.75 ± 0.02 | 0.87 ± 0.01 | 0.93 ± 0.03 |
| WR (kΩ cm2) | 127.3 ± 10.3 | |||
| WT (μF cm−2) | 0.26 ± 0.12 | |||
| WP | 0.25 ± 0.01 | |||
| Rcoat (kΩ cm2) | 5.83 ± 0.03 | 64.7 ± 32.4 | ||
| Ccoat: Y (Ω−1 cm−2 sn) | 6.91 ± 0.20 × 10−8 | 1.69 ± 2.28 × 10−7 | ||
| Ccoat: n | 0.54 ± 0.01 | 0.65 ± 0.01 |
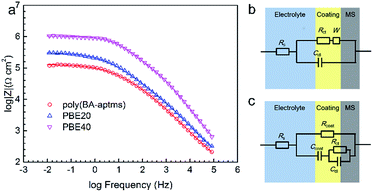
Analyses of EIS plots suggest that different equivalent circuit models are required to fit the results of coated samples, which are shown in Fig. 5. In the case of poly(BA-aptms) coating, a modified Randles EEC is performed (Fig. 5b) with a Warburg impedance element, W,34 which represents the process of the diffusion of ions from the bulk of the electrolyte to the interface and has three parts: WR – ohmic resistance, WT – capacitive part, and WP – its exponent. On the other hand, a second electrochemical interface at the metal/liquid interface is detected by EIS for PBE composite system, which is fitted by a EEC with two time constants showed in Fig. 5c (Rcoat and Ccoat are the coating resistance and capacitance, respectively). The measured and simulated data fit well enough based on the coincidence between the experimental plots and the fitting lines in Fig. 5a. Table 1 presents the electrochemical parameters obtained for bare and coated MS electrodes by EIS data fitting. As expected, the value of the charge transfer resistance, Rct, increases significantly after coating MS with polymer films. It is demonstrated that the Rct value is about 2, 8, 310, and 940 kΩ cm,2 respectively, and an increased ratio of 4, 155, and 570 times for poly(BA-aptms), PBE20, and PBE40 is observed, respectively, compared to the bare MS, which is in good agreement with other electrochemical tests. It is clearly shown that the incorporation of epoxy to poly(BA-aptms) can effectively inhibit the corrosion reaction. However, distinct electrochemical behaviors have been discovered after incorporating epoxy into polybenzoxazine. The corrosive species, especially water molecules diffuse hardly in the polybenzoxazine network due to its hydrophobic nature. So the poly(BA-aptms) coated sample exhibits a high Warburg resistance (above 100 kΩ cm2) with a good barrier ability against the penetration of corrosive ions though its Rct enhancement is much lower than PBE coatings. But the presence of epoxy in polybenzoxazine reduces the hydrophobicity of the coatings, which promotes the electrolyte that has penetrated into the polymeric matrix easily reach the coating/metal interface in comparison to poly(BA-aptms) coated MS. Therefore, a second time constant has been added into the EEC for PBE samples. Nevertheless, the improved crosslinking density by introducing epoxy acts as strong barrier in a corrosive environment, which impedes large amount of electrolyte penetrating into the coating, and that’s the reason why the corrosion resistance of PBE are still higher than poly(BA-aptms). The effects of epoxy on corrosion protection performance of the composite coating will be discussed in the last part of this article.
Hydrophobic stability after corrosive test
Contact angle (CA) measurements were performed to investigate the water wettability of bare and coated MS surfaces before the polarization current experiments and afterward (Fig. 6). All coated samples are hydrophobic with CA values of over 100°. It is obvious that the incorporation of hydrophilic epoxy has little effect on the composite coatings’ surface ability. Moreover, these hydrophobic surfaces keep stable with slight contact angle reduction after the polarization current experiments, indicating that these coatings are hydrophobic in nature, whereas the bare MS surface is substantially hydrophilic with contact angles of about 75° and 70° before and after the test, respectively.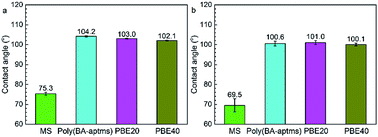 | ||
| Fig. 6 Water contact angles on bare and coated MS surfaces (a) before and (b) after polarization current experiments. | ||
Effects of epoxy on the corrosion resistance of PBE coatings
The enhanced corrosion resistance of polybenzoxazine coatings by epoxy incorporation can be explained through different electrochemical measurements. The features of all test coated samples’ EIS plots (Fig. 5) give an indication of different electrochemical interface inside the coatings, which means that the samples’ anticorrosive performance is determined by their barrier ability and the penetration of corrosive electrolyte (water, oxygen, ion, et al.) is the control procedure for corrosion of metal covered by these coatings. To begin with, as the first obstacle to slow down the penetration of corrosive medium, surface hydrophobicity is an important factor for the coatings’ performance.35 As far as Fig. 6 is concerned, all the coated samples are hydrophobic with the CA values above 100°, which should be attributed to the low surface free energy characteristic of polybenzoxazine. To make matters better, these samples’ surfaces even remain hydrophobic (∼100°) after the corrosion test (Fig. 6b), which is much higher than phenolated oleic acid based polybenzoxazine coated samples investigated by Bălănucă, et al. (80–85°).10 This stability can be an attractive advantage for their future application under prolonged contact with aggressive electrolytes.Preceding this, the diffusion process of corrosive species inside the coatings decides the coatings’ protection performance, which is related tightly to the incorporation of epoxy in the composite system. Fig. 7 illustrates the effects of epoxy on this process. Because water molecules can still pass through the coatings’ surface since micro pores definitely exist in all kinds of organic film, denser polymer network with fewer micro pores should reduce the opportunity of water molecules’ permeation. In accordance with about ten times increased Rcoat (∼60 kΩ cm2) showed by the sample with 40 wt% epoxy compared to that of PBE20 sample (∼6 kΩ cm2) in the EIS results (Table 1), it is worth of noting that the sample showing the higher performance among the studied samples also characterized by the higher content of epoxy, where more opportunities for unoccupied phenolic hydroxyl groups react to epoxide groups to form more dense polymer network when the content of epoxy increases, which is in good agreement with Lin’s work by incorporating amine-capped aniline trimer into polybenzoxazine.13 It is can also be concluded by comparing with other available reported results. The PBE40 coated MS surface exhibit good corrosion protection performance with corrosion current of <10−7 A cm−2 (Fig. 3), which is lower than other findings based on pure polybenzoxazine coating (10 μm phenolated oleic acid based polybenzoxazine coating: ∼10−6 A cm−2; 7 μm vegetable oil-based polybenzoxazine coating: 10−6 to 10−7 A cm−2),10,11 even PBE coating is only ∼5 μm thickness in our work. Therefore, it is demonstrated that the epoxy incorporation can significantly improve corrosion resistance of polybenzoxazine coating, which must be attributed to the dense enhancement by the dual crosslinking network of polybenzoxazine and epoxy. Similar results were obtained by other electrochemical techniques. Considering Fig. 2, it is evident that a sample with a high epoxy content not only shows better stability during the immersion time reflected by the less reduction of Eocp compared with neat poly(BA-aptms) but also presents higher Eocp values among the investigated samples. It is possible to note that epoxy incorporation significantly decreases the micro pores inside the coating matrix through enhancing the crosslinking density of the composite polymer network. It is also suggested that the effect of epoxy on the corrosion protection enhancement of polybenzoxazine coatings is to improve their barrier ability against diffusing corrosive species. That is also the reason why the coatings reduce samples’ corrosion current in both anodic and cathodic direction without selectivity in Fig. 3.
 | ||
| Fig. 7 Effects of epoxy on the corrosion protection performance of polybenzoxazine/epoxy composite coating. | ||
But for the penetrated water, the hydrophilic epoxy network has a higher affinity for them than the hydrophobic polybenzoxazine matrix, which makes the epoxy network serve as a path of water molecules passing through the coatings and reaching the coating/metal interface. As a consequence, a second electrochemical interface forms at the interface, leading to different electrochemical behaviour (Fig. 5). Even so, the increased Rct and decreased Y values of Cdl showed by the sample with 20 wt% and 40 wt% epoxy compared with the neat poly(BA-aptms) in Table 1 indicates that corrosion reaction can be effectively suppressed by incorporating epoxy into polybenzoxazine matrix due to the barrier enhancement of epoxy. More specifically, the coating will be closer to a pure capacitance when the index n approaches to 1.0, suggesting the coating performs better corrosion resistance through its strong barrier and dielectric property.36–38 So it is worth emphasising that introduction of epoxy appears to be a good treatment to improve n, which also indicates the barrier improving effect of epoxy.
Therefore, barrier improvement and hydrophobicity reduction for coatings are two paradoxical effects of epoxy on the coatings’ performance. The barrier improvement must play a prominent role in the initial times of the corrosion process for inhibiting the permeation of corrosive medium based on this study. But the hydrophobicity of the coatings’ bulk network is believed to be an essential factor for their performance under prolonged contact with the corrosive environment. More detailed research must be performed and future work aims at the relationship among crosslinking density, hydrophobicity and corrosion resistance of model polybenzoxazine/epoxy hybrid coating under prolonged immersion.
Experimental
Materials
Bisphenol A (BPA, 99% purity) was purchased from Sinopharm Chemical Reagent Co. Ltd., China. 3-Aminopropyltrimethoxysilane (3-APTMOS, 98% purity) was obtained from Diamond Advanced Material of Chemical Inc., China. Epoxy resins (type E-51, 0.53 eq/100 g) were purchased from Shanghai Resin Factory Co., Ltd., China. Paraformaldehyde, chloroform, xylene, n-butanol and other chemicals (99% purity) were from Shanghai Lingfeng Chemical Corp., China. All chemicals were used as received without purification except chloroform, which was purified by distillation over calcium hydride prior to use. The mild steel (MS) (Q235B, composition in wt%: C: 0.12, Mn: 0.32, Cr: 0.035, Si: 0.14, Ni: 0.040, S: 0.010, P: 0.012, Cu: 0.010, Fe: balance) was provided by Lanpec Technologies Co. Ltd., China.Synthesis of benzoxazine monomer
2,2-Bis(3-(trimethoxysilyl)-n-propyl-3,4-dihydro-2H-1,3-benzoxazine)propane (BA-aptms) was synthesized according to the literature.20,21 The obtained BA-aptms was yellowish and viscous with a yield of 85.1%. FTIR (KBr): ν = 1498 (tri-substituted phenyl group); 1231 (s; νas (C–O–C of benzoxazine ring)), 1016 (s; νs (C–O–C of benzoxazine ring)); 1085 (s; νas (Si–O–C)); 931 (oxazine ring). 1H NMR (400 MHz, chloroform-d, δ): 6.75–7.26 (m, 6H, ArH), 4.82 (s, 4H, O–CH2–N), 3.98 (s, 4H, Ph-CH2–N), 3.58 (s, 18H, Si–O–CH3), 2.73 (t, 4H, N–CH2), 1.66 (m, 4H, –CH2–CH2Si), 0.67 (t, 4H, –CH2–Si).Preparation of polybenzoxazine/epoxy coatings
The substrates (60 mm × 60 mm × 2 mm sized mild steel plates) were blasted by a milling machine to remove rust and other foreign matters (Sa 2½), and then degreased with acetone prior to dip coating. BA-aptms monomer with or without epoxy were dissolved in a mixed solvent (xylene/n-butanol = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at a concentration of 180 mg mL−1. A sonication treatment was applied until uniform solutions were obtained. Subsequently, MS plates were dip into the mixture with a withdrawing speed of 320 mm min−1 for 1 min × 6 times. The coated samples were cured in one step at 230 °C for 2 h. Samples obtained by adding 20 wt% and 40 wt% epoxy were labelled PBE20 and PBE40, respectively, where the molar ratio between the epoxide groups of epoxy and the phenolic hydroxyl groups of resulted polybenzoxazine would be about 1
3) at a concentration of 180 mg mL−1. A sonication treatment was applied until uniform solutions were obtained. Subsequently, MS plates were dip into the mixture with a withdrawing speed of 320 mm min−1 for 1 min × 6 times. The coated samples were cured in one step at 230 °C for 2 h. Samples obtained by adding 20 wt% and 40 wt% epoxy were labelled PBE20 and PBE40, respectively, where the molar ratio between the epoxide groups of epoxy and the phenolic hydroxyl groups of resulted polybenzoxazine would be about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The thickness of the coatings was evaluated on the stainless steel surfaces before and after coating using a micrometer (Mitutoyo, Japan), resulting on thicknesses of ∼5 μm for all coatings.
1, respectively. The thickness of the coatings was evaluated on the stainless steel surfaces before and after coating using a micrometer (Mitutoyo, Japan), resulting on thicknesses of ∼5 μm for all coatings.
Characterization
Chemical structures of benzoxazine monomer and polymers were investigated by the Fourier transform infrared (FTIR) spectroscopic measurements, which were carried out on a Nicolet iS10 FTIR spectrometer (USA) at room temperature (∼25 °C) using the KBr pellet method, a thin film of the sample was cast from a chloroform solution on a KBr plate. To follow polymerization of the benzoxazines, the cast film on the KBr plate was isothermally cured in an oven under air atmosphere.Static contact angles of the samples were determined by contact angle goniometry (DataPhysicsOCA20, Germany) at ambient conditions through image-capture software by injecting a 2 μL deionized water drop. The reported values are the average of five measurements with the tip not being in contact with the droplet.
Electrochemical measurements were taken with a CH Instruments CHI660D (USA) workstation with a three-electrode system. The coated sample acted as the working electrode, a Ag/AgCl (saturated KCl) electrode was used as the reference electrode and a stainless steel cylinder as the counter electrode. The electrodes working area was ∼14 cm2. All tests were performed in a corrosive medium (3.5 wt% NaCl aqueous solution) at ambient temperature. Samples were immersed for 30 min to ensure steady-state prior to measurements, and measurements were repeated at least three times. In the polarization current experiments, the potential was scanned from −100 mV below to +100 mV vs. Ag/AgCl above the corrosion potential Ecorr at a scan rate of 2 mV s−1. In the electrochemical impedance spectroscopy (EIS) measurements, a sinusoidal AC perturbation of 10 mV amplitude coupled with an open circuit potential was applied to the metal/coating system. The EIS test was managed in the frequency range from 100 kHz to 10 mHz. EIS analysis was performed by using Zview software.
Conclusions
Corrosion resistance of the polybenzoxazine (poly(BA-aptms)) coating was improved by incorporating epoxy into the coatings’ matrix. FTIR results confirm the formation of a dual crosslinking network of polybenzoxazine and epoxy. Effects of epoxy incorporation on the coatings’ performance were fully investigated by electrochemical measurements and water contact angle tests. It is found that corrosion protection performance of composite coatings is enhanced with increasing epoxy content due to the possible increased crosslinking density of coatings’ matrix by the increscent consumption of unoccupied phenolic hydroxyl groups of polybenzoxazine. Otherwise, good hydrophobic surface stability can also be obtained for all investigated coated samples, which is a promising feature for future practical application.Acknowledgements
The authors gratefully acknowledge the financial from Program of Shanghai Subject Chief Scientist (10XD1401500), Program of Leading Talents (2013), National Natural Science Foundation of China (21506062), Fundamental Research Funds for the Central Universities (22A201514015), China Postdoctoral Science Foundation (2015M571509), Shanghai Key Laboratory of Multiphase Materials Chemical Engineering (MMCE2015004) and Lanpec Technologies Co., Ltd (China).References
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344–1391 CrossRef CAS.
- Y. Yagci, B. Kiskan and N. N. Ghosh, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5565–5576 CrossRef CAS.
- C. P. R. Nair, Prog. Polym. Sci., 2004, 29, 401–498 CrossRef CAS.
- H. Ishida and T. Agag, Handbook of benzoxazine resins, Elsevier, 2011 Search PubMed.
- A. Van, K. Chiou and H. Ishida, Polymer, 2014, 55, 1443–1451 CrossRef CAS.
- U. Thubsuang, H. Ishida, S. Wongkasemjit and T. Chaisuwan, J. Mater. Sci., 2014, 49, 4946–4961 CrossRef CAS.
- K. Zhang, R. Cai, Q. Zhuang, X. Liu, G. Yang and Z. Han, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1514–1518 CrossRef CAS.
- J. Escobar, M. Poorteman, L. Dumas, L. Bonnaud, P. Dubois and M.-G. Olivier, Prog. Org. Coat., 2015, 79, 53–61 CrossRef CAS.
- C. Zhou, X. Lu, Z. Xin and J. Liu, Corros. Sci., 2013, 70, 145–151 CrossRef CAS.
- B. Bălănucă, M. Raicopol, A. Maljusch, S. Garea, A. Hanganu, W. Schuhmann and C. Andronescu, ChemPlusChem, 2015, 80, 1170–1177 CrossRef.
- M. Raicopol, B. Bălănucă, K. Sliozberg, B. Schlüter, S. A. Gârea, N. Chira, W. Schuhmann and C. Andronescu, Corros. Sci., 2015, 100, 386–395 CrossRef CAS.
- B. Kiskan, N. N. Ghosh and Y. Yagci, Polym. Int., 2011, 60, 167–177 CrossRef CAS.
- S.-C. Lin, C.-S. Wu, J.-M. Yeh and Y.-L. Liu, Polym. Chem., 2014, 5, 4235–4244 RSC.
- C. Zhou, X. Lu, Z. Xin, J. Liu and Y. Zhang, Corros. Sci., 2014, 80, 269–275 CrossRef CAS.
- X. Shi, T. A. Nguyen, Z. Suo, Y. Liu and R. Avci, Surf. Coat. Technol., 2009, 204, 237–245 CrossRef CAS.
- B. Ramezanzadeh and M. M. Attar, Prog. Org. Coat., 2011, 71, 314–328 CrossRef CAS.
- S. Grishchuk, S. Schmitt, O. C. Vorster and J. Karger-Kocsis, J. Appl. Polym. Sci., 2012, 124, 2824–2837 CrossRef CAS.
- S. Rimdusit, P. Kunopast and I. Dueramae, Polym. Eng. Sci., 2011, 51, 1797–1807 CAS.
- W. Li, H. Tian and B. Hou, Mater. Corros., 2012, 63, 44–53 CrossRef CAS.
- H. Ishida and H. Y. Low, J. Appl. Polym. Sci., 1998, 69, 2559–2567 CrossRef CAS.
- Y. Liu, W. Zhang, Y. Chen and S. Zheng, J. Appl. Polym. Sci., 2006, 99, 927–936 CrossRef CAS.
- H. Ishida and D. J. Allen, Polymer, 1996, 37, 4487–4495 CrossRef CAS.
- H. Kimura, A. Matsumoto, K. Hasegawa, K. Ohtsuka and A. Fukuda, J. Appl. Polym. Sci., 1998, 68, 1903–1910 CrossRef CAS.
- S. Rimdusit and H. Ishida, Polymer, 2000, 41, 7941–7949 CrossRef CAS.
- C. H. Lin, C. M. Huang, T. I. Wong, H. C. Chang, T. Y. Juang and W. C. Su, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1359–1367 CrossRef CAS.
- M. A. Espinosa, M. Galia and V. Cadiz, Polymer, 2004, 45, 6103–6109 CrossRef CAS.
- M. G. González, J. Baselga and J. C. Cabanelas, Infrared Spectroscopy – Materials Science, Engineering and Technology, INTECH Open Access Publisher, 2012, pp. 261–270 Search PubMed.
- W.-G. Ji, J.-M. Hu, J.-Q. Zhang and C.-N. Cao, Corros. Sci., 2006, 48, 3731–3739 CrossRef CAS.
- B. Nikravesh, B. Ramezanzadeh, A. A. Sarabi and S. M. Kasiriha, Corros. Sci., 2011, 53, 1592–1603 CrossRef CAS.
- S. Radhakrishnan, C. R. Siju, D. Mahanta, S. Patil and G. Madras, Electrochim. Acta, 2009, 54, 1249–1254 CrossRef CAS.
- Z. Tao, S. Zhang, W. Li and B. Hou, Corros. Sci., 2009, 51, 2588–2595 CrossRef CAS.
- P. C. Okafor, X. Liu and Y. G. Zheng, Corros. Sci., 2009, 51, 761–768 CrossRef CAS.
- H. H. Hassan, E. Abdelghani and M. A. Amin, Electrochim. Acta, 2007, 52, 6359–6366 CrossRef CAS.
- D. Prasai, J. C. Tuberquia, R. R. Harl, G. K. Jennings and K. I. Bolotin, ACS Nano, 2012, 6, 1102–1108 CrossRef CAS PubMed.
- O. U. Rahman and S. Ahmad, RSC Adv., 2014, 4, 14936–14947 RSC.
- S.-D. Xu, Q.-C. Zhuang, L.-L. Tian, Y.-P. Qin, L. Fang and S.-G. Sun, J. Phys. Chem. C, 2011, 115, 9210–9219 CAS.
- M. Qian, A. McIntosh Soutar, X. H. Tan, X. T. Zeng and S. L. Wijesinghe, Thin Solid Films, 2009, 517, 5237–5242 CrossRef CAS.
- M. Fedel, E. Callone, S. Diré, F. Deflorian, M. G. Olivier and M. Poelman, Electrochim. Acta, 2014, 124, 90–99 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |