Supercapacitors based on polyelectrolyte/ferrocenyl-surfactant complexes with high rate capability
Guiping
Tan
a,
Zhiyu
Cheng
*a,
Yongfu
Qiu
*a,
Weiqing
Huang
a,
Hongbo
Fan
a and
Biye
Ren
b
aCollege of Chemistry and Environmental Engineering, Guangdong Engineering and Technology Research Center for Advanced Nanomaterials, Dongguan University of Technology, Guangdong 523808, P. R. China. E-mail: qiuyf1979@foxmail.com; mszycheng@scut.edu.cn; Fax: +86 769 2286 1232; Tel: +86 769 2286 1232
bResearch Institute of Materials Science, South China University of Technology, Guangzhou 510640, P. R. China
First published on 23rd March 2016
Abstract
In this paper, a composite of the Polyelectrolyte/Ferrocenyl-Surfactant Complexes (PFSC) coated on carbon paper (CP) was applied as an electrode material for supercapacitors. The as-prepared composite possessed a specific capacitance as high as 214 F g−1 calculated from discharge curve with a current density of 0.8 A g−1 and this observation was deemed to be because of its ferrocenyl moieties. Furthermore, the specific capacitances calculated from cyclic voltammograms were 145 and 103 F g−1 at the potential scan rates of 20 and 50 mV s−1, which kept at 77.6% and 55.4% of the corresponding value at 10 mV s−1 (187 F g−1). This suggested that the composite possessed a good rate capability as a novel redox active electrode material for pseudo-capacitors. In short, for the first time the composite PFSC/CP was reported as a promising electrode material for efficient supercapacitors.
1. Introduction
Supercapacitors with high power and a much longer shelf and cycle life than batteries have attracted great attention over the past few years.1–3 Generally, electrical double layer capacitors (EDLCs) store energy in terms of an electrical double-layer. To enhance their capacitances, a typical strategy is to utilize carbon electrodes with large surface areas and appropriate pore-size distributions. Carbon materials including activated carbon, carbon nanotube, carbon spheres, porous carbon and graphene are usually used as electrodes.1–9 In comparison with EDLCs, pseudo-capacitors are described in terms of faradic charge transfer originating by a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes. They are more attractive due to their higher capacitance and energy density through faradic reactions.1–3,10 Manganese dioxide (MnO2) is considered as one of the most promising pseudo-capacitive materials because of its superior capacitance (>1370 F g−1), environment friendly and cost-effective characteristics.11 However, pseudo-capacitors based on MnO2 often provide poor rate capability due to its low electrical conductivity (10−5 to 10−6 S cm−1).12,13In order to enhance rate capability for pseudo-capacitors, other electrode materials with redox couple could be considered. Ferrocene and its derivatives have been used as a family of fascinating organometallic electron mediators because of their redox properties. They usually serve as the electron transfer media between redox matrix and electrode in electrochemical and biological sensors.14–17 Therefore, Polyelectrolyte/Ferrocenyl-Surfactant Complexes (PFSC) are expected to be used as electrode materials in pseudo-capacitors, due to their excellent redox properties originating from that the generated electrons on ferrocenyl groups will be conducted and separated through self-assembled and -organized 3D structures.
In this research, the PFSC was used as an electrodes material for pseudo-capacitors and the commercial carbon papers was used as substrates and current collectors. The PFSC were prepared via the ionic self-assembly of sodium poly(styrenesulfonate) (PSS) and (11-ferrocenylundecyl)-trimethylammonium bromide (FTMA) in aqueous solution. PFSC film was anchored onto the carbon paper (CP) to form a composite PFSC/CP through a solution casting technique. The as-prepared composite possesses a specific capacitance as high as 214 F g−1 calculated from discharge curve with current density 0.80 A g−1. Furthermore, the specific capacitances calculated from cyclic voltammograms are 145 and 103 F g−1 at potential scan rate 20 and 50 mV s−1, which retain 77.6% and 55.4% of the corresponding value at 10 mV s−1 (187 F g−1). This indicates that the PFSC/CP composite possesses good rate capability as a novel redox active electrode material for pseudo-capacitors.
2. Experimental
2.1 Materials
Sodium poly(styrenesulfonate) (PSS, Aldrich, MW = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was used as received. 11-Bromoundecyl ferrocene was synthesized according to our previous report.18 All the other chemicals were analytical reagents and purified according to the standard procedures. Water used in all experiments was deionized and filtrated by a Millipore purification apparatus with resistivity more than 18.0 MΩ cm.
000) was used as received. 11-Bromoundecyl ferrocene was synthesized according to our previous report.18 All the other chemicals were analytical reagents and purified according to the standard procedures. Water used in all experiments was deionized and filtrated by a Millipore purification apparatus with resistivity more than 18.0 MΩ cm.
2.2 Synthesis of (11-ferrocenylundecyl)trimethylammonium bromide (FTMA)
The ferrocenyl surfactant FTMA was synthesized according to the literature procedure.19 The target product FTMA was synthesized through uncleophilic substitution reaction between 11-bromoundecyl ferrocene and trimethylamine. The synthetic routes and 1H NMR spectrum of the FTMA measured in CDCl3 are shown in Fig. 1 and 2, respectively. The characterization data are listed as follows, 1H NMR (CDCl3, TMS) δ (ppm) 4.11 (m, 9H, H(Cp)), 3.54 (t, 2H, –CH2–N–), 3.40 (s, 9H, (CH3)3–N–), 2.23 (t, 2H, Cp–CH2–), 1.69 (m, 2H, –CH2–CH2–N–), 1.44–1.23 (m, 16H, Cp–CH2–CH2–(CH2)8–). FT-IR (KBr) ν 3092, 1466, 1105, 1000, and 816 cm−1 (characteristics of ferrocene groups absorption peaks), 2923 cm−1 (νas CH2), 2852 cm−1 (νs CH2), 1631 cm−1 (νs![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+
N+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ).
).
2.3 Preparation of PFSC and its film
The PFSC was prepared according to the following procedure: 239 mg (0.5 mmol) of FTMA was dissolved in 5 mL of water at about 80 °C and slowly added dropwise into 5 mL of boiling water containing 103 mg (0.5 mmol of repeat unit) of PSS under stirring. The yellow precipitate was separated by filtration, washed several times with hot water, and dried for 76 h under vacuum at 40 °C. The chemical structure of PFSC and its electron transfer reaction equation were illustrated in Fig. 3. For preparation of complex films, the chloroform solution of the PFSC with 2 × 10−5 mol L−1 (counted for the ferrocenyl surfactant, the following is the same) was coated on a quartz glass at room temperature and dried for 48 h under vacuum at 40 °C.2.4 Preparation of PFSC/CP
As a general procedure, carbon paper (CP) electrode was sonicated in ethanol and doubly distilled water for 30 s, respectively. A freshly cleaned CP electrode was soaked in a solution of 0.07 mol L−1 PFSC in chloroform for 10 s. A small bottle was sealed tightly covering the electrode to serve as a closed chamber to make chloroform evaporate slowly. The CP electrode modified with the PFSC film (denoted as PFSC/CP) was then dried in N2 atmosphere at room temperature. The loading mass of the PFSC for PFSC/CP electrode are 1.0 mg cm−2.2.5 Characterization
Wide-angle X-ray diffraction (WAXD) and small-angle X-ray scattering (SAXS) measurements of the complex film were performed in transmission geometry with an X'pert PRO diffractometer (40 KV and 40 mA) using Cu Kα radiation (wavelength λ = 0.154 nm) at room temperature. The 2θ ranged from 10° to 30° for WAXD and the scattering vector s ranged from 0.1 to 1.5 nm−1 for SAXS, where s = (2/λ)sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. The scan step was 0.01° in 2θ with counting time of 1 s per step. Thermogravimetry (TG) was measured with a Netzsch TG 209 under nitrogen atmosphere at heating rate of 10 °C min−1 starting from room temperature up to 750 °C. The differential scanning calorimetry (DSC) was carried out with 4–5 mg of sample in a 6 mm aluminum pan on a Netzsch DSC 204 under nitrogen atmosphere at heating or cooling rate of 10 °C min−1 following the temperature sequence as room temperature → 130 °C → −45 °C → 130 °C. Polarized optical microscope of Zeiss Axiophot was used with a Linkam hot stage. 1H NMR spectrum was obtained in CDCl3 solution on a Varian INOVA 500NB spectrometer. UV-visible absorbance was measured with a Hitachi UV-3010 spectrophotometer. Polarized optical microscope (POM) of Zeiss Axiophot was used with a Linkam hot stage. The sample was heated from room temperature to 110 °C to remove the heat history and then cooled to room temperature and subsequently heated to 110 °C again at the rate of 10 °C min−1. The POM photos of the PFSC were taken at 25 and 100 °C for smectic A phase and isotropic state, respectively. Cyclic voltammetry (CV) and galvanostatic charge/discharge measurements were performed in a three-electrode system using a CHI 440a electrochemical work station, with 0.1 M Na2SO4 as the electrolyte solution. The composite PFSC/CP with a projection area of 0.2 cm2 were used as the working electrode, Ag/AgCl (3.0 M KCl) and a Pt wire as the reference and counter electrodes, respectively. The electrochemical impedance spectroscopy (EIS) measurements were carried out by using a potentiostat (EG&G, M2273), the frequency range analyzed was 0.1 Hz to 100 Hz with AC amplitude of 10 mV.
θ. The scan step was 0.01° in 2θ with counting time of 1 s per step. Thermogravimetry (TG) was measured with a Netzsch TG 209 under nitrogen atmosphere at heating rate of 10 °C min−1 starting from room temperature up to 750 °C. The differential scanning calorimetry (DSC) was carried out with 4–5 mg of sample in a 6 mm aluminum pan on a Netzsch DSC 204 under nitrogen atmosphere at heating or cooling rate of 10 °C min−1 following the temperature sequence as room temperature → 130 °C → −45 °C → 130 °C. Polarized optical microscope of Zeiss Axiophot was used with a Linkam hot stage. 1H NMR spectrum was obtained in CDCl3 solution on a Varian INOVA 500NB spectrometer. UV-visible absorbance was measured with a Hitachi UV-3010 spectrophotometer. Polarized optical microscope (POM) of Zeiss Axiophot was used with a Linkam hot stage. The sample was heated from room temperature to 110 °C to remove the heat history and then cooled to room temperature and subsequently heated to 110 °C again at the rate of 10 °C min−1. The POM photos of the PFSC were taken at 25 and 100 °C for smectic A phase and isotropic state, respectively. Cyclic voltammetry (CV) and galvanostatic charge/discharge measurements were performed in a three-electrode system using a CHI 440a electrochemical work station, with 0.1 M Na2SO4 as the electrolyte solution. The composite PFSC/CP with a projection area of 0.2 cm2 were used as the working electrode, Ag/AgCl (3.0 M KCl) and a Pt wire as the reference and counter electrodes, respectively. The electrochemical impedance spectroscopy (EIS) measurements were carried out by using a potentiostat (EG&G, M2273), the frequency range analyzed was 0.1 Hz to 100 Hz with AC amplitude of 10 mV.
2.6 Calculation
The specific capacitance for the electrode can be calculated as, , where C is the specific capacitance (F g−1), m is the mass of PFSC (g), υ is the potential scan rate (V s−1), Uc − Ua is the sweep potential range during (U) discharging branch and I(U) denotes the response current density (A g−1). In addition, gravimetric capacitance for a single electrode was calculated from the discharge curve in the three-electrode cell by the equation,
, where C is the specific capacitance (F g−1), m is the mass of PFSC (g), υ is the potential scan rate (V s−1), Uc − Ua is the sweep potential range during (U) discharging branch and I(U) denotes the response current density (A g−1). In addition, gravimetric capacitance for a single electrode was calculated from the discharge curve in the three-electrode cell by the equation,  , where I is the constant current (A g−1), Δt is the discharge time, ΔV is the voltage range during the discharge process.20
, where I is the constant current (A g−1), Δt is the discharge time, ΔV is the voltage range during the discharge process.20
3. Results and discussion
3.1 Structural analysis
WAXD and SAXS measurements were performed and shown in Fig. 4 for PFSC film at room temperature. In the wide angle range (Fig. 4A), only a broad peak appeared at 2θ ≈ 17.7°, corresponding to the distance between the conformationally disordered alkyl chains. This indicated the non-crystalline structure in the solid PFSC at room temperature. | ||
Fig. 4 WAXD (A) and SAXS (B) patterns of the PFSC, where s = (2/λ)sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ; (C) possible structure model of the PFSC. θ; (C) possible structure model of the PFSC. | ||
In the small angle range (Fig. 4B), there were two peaks at the equidistant position in s scale as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (the scattering vectors s of 0.32 and 0.63 nm−1 correspond to the long period of d001 = 31.3 and d002 = 15.8 Å, respectively), indicating the typical lamella mesomorphous structure for PFSC. The results were similar to that proposed by Antonietti et al. for the complexes of PSS and different alkyltrimethyammonium derivatives.21 They suggested that the lamella was composed of two layers as polyions (polyelectrolyte and ionic head groups) and alkyl chains (tails) and the long period depends on the length and orientation of alkyl chains of surfactant. With the results of Antonietti et al.,21 the thickness of the alkyl tail layer was estimated to be 17.3 Å for PFSC. The value was close to the tail length (∼18.8 Å) calculated for the stretched corresponding alkyl chains with the given carbon numbers and ferrocenyl groups (∼5 Å), because the increment of one carbon is about 1.25 Å to the long period for a completely stretched alkyl chain if the chain was perpendicular to the lamella.21 Hence, the surfactant tails were interdigitately arranged in a monolayer in the complexes. The possible structure of PFSC film is schematically illustrated in Fig. 4C.
2 (the scattering vectors s of 0.32 and 0.63 nm−1 correspond to the long period of d001 = 31.3 and d002 = 15.8 Å, respectively), indicating the typical lamella mesomorphous structure for PFSC. The results were similar to that proposed by Antonietti et al. for the complexes of PSS and different alkyltrimethyammonium derivatives.21 They suggested that the lamella was composed of two layers as polyions (polyelectrolyte and ionic head groups) and alkyl chains (tails) and the long period depends on the length and orientation of alkyl chains of surfactant. With the results of Antonietti et al.,21 the thickness of the alkyl tail layer was estimated to be 17.3 Å for PFSC. The value was close to the tail length (∼18.8 Å) calculated for the stretched corresponding alkyl chains with the given carbon numbers and ferrocenyl groups (∼5 Å), because the increment of one carbon is about 1.25 Å to the long period for a completely stretched alkyl chain if the chain was perpendicular to the lamella.21 Hence, the surfactant tails were interdigitately arranged in a monolayer in the complexes. The possible structure of PFSC film is schematically illustrated in Fig. 4C.
The chromophores could stack in parallel and form an angle α between the chromophore transition dipoles and the line passing through the dipole centers. For the H-aggregate α was greater than 54.7° and for the J-aggregate α was less than 54.7°.22Fig. 5 showed UV-vis spectra of the PFSC in film and in chloroform solution. There were usually two peaks at ca. 240 and 440 nm in the UV-vis spectrum for the ferrocene and its derivatives, but the latter was very weak. As seen from Fig. 5, the maximum absorbance of the PFSC in chloroform solution appeared at about λmax = 240 nm, resulting from the π–π* electron transition of ferrocenyl moieties. In contrast, the PFSC film presented λmax at about 203 nm, which blue-shifts about 37 nm from λmax = 240 nm for the PFSC in solution, suggesting the H-aggregate among ferrocenyl moieties causing an increase in the π–π* transfer energy of cyclopentadiene, similar to that reported by Shen et al.23 on the N,N-dimethylferrocenyl methylhexadecyl-ammonium (Fc16) bromide containing LB film. According to exciton-coupling theory developed by Kasha et al.,22 noncovalent dimerization of molecules within an aggregate lowers the degeneracy of its excited electronic state compared to the uncoupled monomers. For H-aggregates, the higher electronic state carries all of the oscillating strength while transition to the lower electronic (exciton) state is forbidden. As the result, the absorption of the aggregate is blue-shifted in accordance with Kasha's rules.24 The present behavior suggests that the ferrocenyl moieties of the PFSC film would form a close molecular packing as the H-aggregate, i.e., the cyclopentadienyl rings of ferrocenyl moieties are parallel to each other and tilted in the complex film organized by the ordered packing structure of alkyl tails. The H-aggregate among ferrocenyl moieties of PFSC film is also schematically illustrated in Fig. 4C.
 | ||
| Fig. 5 UV-vis spectra of the PFSC in chloroform solution (a) and solid film (b). Inset is the H-aggregate model for ferrocenyl moieties in the PFSC. | ||
3.2 Thermal properties and POM analysis
In order to understand the nature of PFSC, we determined the thermal properties with TG and DSC. TG thermograms in Fig. 6A demonstrate that there is no significant weight-loss up to 220 °C for PFSC, indicating their high thermal stability. PFSC exhibited a two-step weight-loss process over 220–750 °C. The degradation temperature Td1 corresponding to the first maximum weight-loss rate for the PFSC is 338 °C, while degradation temperature Td for the surfactants FTMA is approximately 265 °C. This indicates that the thermal stability of the complex is significantly enhanced when compared with the corresponding pure surfactant as the result of strong electrostatic interaction between –SO3− (from PSS) and![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N+
N+![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) (from ferrocenyl surfactant). The second maximum weight-lost at about 460 °C is attributed to the degradation of PSS polymer in the complex. The corresponding degradation temperature Td2 increases from 442 °C for the pure PSS to 460 °C for the complexes. In a word, according to the higher degradation temperature, the complexes showed higher thermal stability compared with their components due to the ionic interaction.
(from ferrocenyl surfactant). The second maximum weight-lost at about 460 °C is attributed to the degradation of PSS polymer in the complex. The corresponding degradation temperature Td2 increases from 442 °C for the pure PSS to 460 °C for the complexes. In a word, according to the higher degradation temperature, the complexes showed higher thermal stability compared with their components due to the ionic interaction.
Only a weak endothermic peak at 93.6 °C and no glass transition during the second heating run were detected from the DSC trace above −45 °C for PFSC (Fig. 6B). There was no glass transition in complexes due to the stiffening of the PSS chains resulting from arrangement of the bound FTMA. We considered that the complexes were in the liquid crystal state below Ti (93.6 °C) corresponding to the transition from liquid crystal to isotropic phase as further verified with POM. Fig. 6C and D illustrated POM photographs of the complexes taken at 25 and 100 °C, respectively. PFSC exhibited a random birefringent polygon-like texture below Ti (Fig. 6C). The oriented texture was observed after sheared and disappeared when temperature was above Ti (Fig. 6D). Combining with the finding from WAXD and SAXS, PFSC was ionic thermotropic smectic A phase below Ti.
3.3 Cyclic voltammetry and galvanostatic charge/discharge measurements
In order to examine the electrochemical behaviors of our samples, their cyclic voltammograms and galvanostatic charge/discharge results are shown in Fig. 7. In Fig. 7A, the CV curve of CP with quasi-rectangular was observed exhibiting their double-layer capacitance behaviors, and a couple of redox peaks were examined from CV curve of PFSC/CP, indicating this composite with obviously redox behaviours of PFSC, which similar with other ferrocenyl-modified materials.To investigate the electrochemical effect of PFSC on the composite PFSC/CP, the areal capacitances for CP and PFSC/CP at scan rate 10 mV s−1 are calculated as 38 and 60 mF cm−2 shown in Fig. 7B. It should be noted that their areal capacitances calculated using the equation:  ,20 where S is the plane area of sample. The calculated results suggest that PFSC anchored onto CP has dramatically increased the areal capacitances.
,20 where S is the plane area of sample. The calculated results suggest that PFSC anchored onto CP has dramatically increased the areal capacitances.
The typical CV curves for PFSC/CP at different potential scan rates are given in Fig. 7C and they all have a couple of redox peaks at 10, 20 and 50 mV s−1 potential scan rates. This indicates that the composite PFSC/CP has redox properties and excellent rate capabilities.1 While, the CV curves deviating from redox peaks shape upon potential scan rates higher than 50 mV s−1 imply that the CV processes were controlled by the relatively slow diffusion of ions in electrolyte such as Na+, SO42−, H+ and OH− or by these ions diffusion in the two-stage structure of PFSC/CP, and accordingly some PFSC materials under the interface does not make a remarkable contribution for the capacitance performance of pseudo-capacitors.25,26 The specific capacitances of PFSC/CP calculated from cyclic voltammograms are shown in Fig. 7D. The decrease of the specific capacitances for PFSC/CP with the increasing potential scan rates can be observed. Their specific capacitances at scan rates of 20 and 50 mV s−1 are 145 and 103 F g−1 respectively, which retain 77.6% and 55.4% of the corresponding value 187 F g−1 at 10 mV s−1. This implies that the composite PFSC/CP has excellent rate capability as an electrode material for supercapacitors.
The PFSC/CP exhibiting the best supercapacitor performance was confirmed by the galvanostatic charge/discharge measurements in the three-electrode system. In Fig. 8A, the charge–discharge curves for PFSC/CP at different current densities generally show symmetrical and linear profiles upon current densities larger than 2.4 A g−1, suggesting its excellent supercapacitive behaviors. However, once current densities lower than 1.6 A g−1, an obvious platform could be clearly observed in charge or discharge curve, which is due to the reversibility reaction of oxidation/reduction for the ferrocenyl motifs in the composite PFSC.
 | ||
| Fig. 8 (A) Charge–discharge curves at various current densities for PFSC/CP; (B) areal capacitance calculated based on charge–discharge curves from plot (A) as a function of current density. | ||
The typically specific capacitances for a single electrode calculated from the discharge branches are shown in Fig. 8B. Discharging at varied specific current densities 0.8, 1.6, 2.4, and 3.2 A g−1, the corresponding specific capacitances are 214, 132, 62 and 31 F g−1. This also implies the excellent supercapacitive behaviors of the composite PFSC/CP in terms of superior reversible redox reactions taking place within the sample. At a current density 0.80 A g−1, the calculated specific capacitance for PFSC/CP is 214 F g−1. This value is close to 250.0 F g−1 for the flat MnO2 electrode.27 In addition to high rate capability, the cycle life test over 2000 cycles for PFSC/CP was performed at a current density of 3.2 A g−1 and the results in Fig. 9A showed that the capacitance decay over 2000 cycles is ∼12%, indicating its long cycle life.
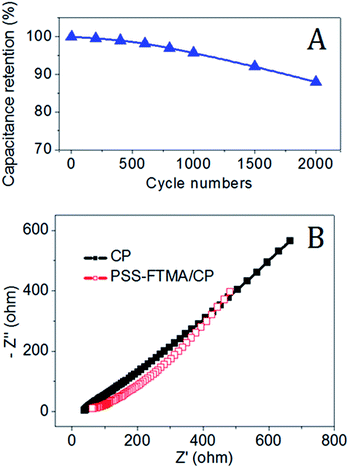 | ||
| Fig. 9 (A) Capacitance retention test over 2000 cycles at a current density of 1.0 A g−1 for PFSC/CP; (B) Nyquist electrochemical impedance spectra of CP and PFSC/CP. | ||
The power capability of supercapacitors is primarily dependent on their resistance, according to the equationPsc = 9(1 − EF)Vo2/16Rsc, where Vo is the rated voltage of the supercapacitor, Rsc is the resistance of the supercapacitor and EF is the efficiency of the supercapacitor.28 Usually, the resistance response is measured in the pulsed mode of operation for determining the peak useable power capability. The Nyquist-type impedance spectra in Fig. 9B show that only upward sloping lines for CP and PFSC/CP electrodes, which suggesting that both electrodes have low diffusion resistance and exhibit high power capacitive performance.29,30
To design high performance supercapacitors utilizing pseudo-capacitance, the electronic conductivity and redox property of electrode materials are the two facts playing the key roles in affecting the electrochemical behaviours. In our case, the carbon paper has well hydrophobility leading to its strong interaction with the deposited PFSC, thus with low resistance response for electrodes. In addition, the redox properties of PFSC are advantageous to improve the specific capacitance for the electrode. In brief, the composite PFSC/CP was reported for the first time as a promising electrode material for efficient supercapacitors.
4. Conclusion
In summary, before preparing the composite PFSC/CP, the redox active polyelectrolyte-surfactant complexes PFSC were prepared via the ionic self-assembly firstly. The PFSC complex exhibited an ordered interdigitated monolayer mesomorphous structure with the long period of d = 3.13 nm, and was in the ionic thermotropic liquid crystal SmA state at room temperature. Interestingly, in the complex film, the ferrocenyl moieties formed H-aggregation. Furthermore, the complexes showed higher thermal stability compared with their components. The electrochemical behaviors of the composite PFSC/CP were characterized by cyclic voltammograms and galvanostatic charge/discharge. The composite PFSC/CP possessed a specific capacitance as high as 214 F g−1. Their specific capacitances at scan rates of 20 and 50 mV s−1 are 145 and 103 F g−1 respectively, which retain 77.6% and 55.4% of the corresponding value 187 F g−1 at 10 mV s−1. The high capacitance and high rate capability of our supercapacitors originated from the low resistance response and the very fast reversible faradaic redox of the composite PFSC/CP. In a word, the composite PFSC/CP is reported for the first time, and it is a promising electrode material for building up an efficient supercapacitor.Acknowledgements
The work described in this paper was supported by the National Natural Science Foundation of China (No. 21274047), the characteristic innovation project for natural science in Guangdong provincial key platform and major scientific research projects for colleges and universities (No. 2015KTSCX139), foundation for the Excellent Young Teachers in Higher Education Institutions of Guangdong (Yongfu Qiu, 2014), the Natural Science Foundation of Guangdong Province (No. 2015A030313651, 2015A030310235), the Foundation of the Department of Education of Guangdong Province (No. 2014KQNCX218). Thanks to Qiu Yan (1941308558@qq.com) for her linguistic assistance during the preparation of this manuscript.Notes and references
- G. P. Wang, L. Zhang and J. J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- D. S. Yu, Q. H. Qian, L. Wei, W. C. Jiang, K. L. Goh, J. Wei, J. Zhang and Y. Chen, Chem. Soc. Rev., 2015, 44, 647–662 RSC.
- X. Peng, L. L. Peng, C. Z. Wu and Y. Xie, Chem. Soc. Rev., 2014, 43, 3303–3323 RSC.
- Y. W. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- P. H. Jampani, O. Velikokhatnyi, K. Kadakia, D. H. Hong, S. S. Damle, J. A. Poston, A. Manivannan and P. N. Kumta, J. Mater. Chem. A, 2015, 3, 8413–8432 CAS.
- X. L. Chen, H. J. Lin, P. N. Chen, G. Z. Guan, J. Deng and H. S. Peng, Adv. Mater., 2014, 26, 4444 CrossRef CAS PubMed.
- A. M. Abdelkader, J. Mater. Chem. A, 2015, 3, 8519–8525 CAS.
- W. Q. Tian, Q. M. Gao, Y. L. Tan, K. Yang, L. H. Zhu, C. X. Yang and H. Zhang, J. Mater. Chem. A, 2015, 3, 5656–5664 CAS.
- J. H. Shi, X. C. Li, G. H. He, L. Zhang and M. Li, J. Mater. Chem. A, 2015, 3, 20619–20626 CAS.
- K. Zhang, X. P. Han, Z. Hu, X. L. Zhang, Z. L. Tao and J. Chen, Chem. Soc. Rev., 2015, 44, 699–728 RSC.
- Z. B. Lei, J. T. Zhang and X. S. Zhao, J. Mater. Chem., 2012, 22, 153–160 RSC.
- F. Cao, Y. M. Liu, B. L. Chen, L. F. Fei, Y. Wang and J. K. Yuan, Electrochim. Acta, 2012, 81, 1–7 CrossRef CAS.
- S. W. Lee, J. Kim, S. Chen, P. T. Hammond and Y. Shao-Horn, ACS Nano, 2010, 4, 3889–3896 CrossRef CAS PubMed.
- M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935–1949 CrossRef CAS PubMed.
- F. Li, Y. Q. Yu, Q. Li, M. Zhou and H. Cui, Anal. Chem., 2014, 86, 1608–1613 CrossRef CAS PubMed.
- K. W. Ren, J. Wu, F. Yan, Y. Zhang and H. X. Ju, Biosens. Bioelectron., 2015, 66, 345–349 CrossRef CAS PubMed.
- C. Arivazhagan, R. Borthakur and S. Ghosh, Organometallics, 2015, 34, 1147–1155 CrossRef CAS.
- C. Li, B. Ren, Y. Zhang, Z. Cheng, X. Liu and Z. Tong, Langmuir, 2008, 24, 12911–12918 CrossRef CAS PubMed.
- T. Saji, K. Hoshino, Y. Ishii and M. Goto, J. Am. Chem. Soc., 1991, 113, 450–456 CrossRef CAS.
- J. Yan, Z. J. Fan, T. Wei, J. Cheng, B. Shao, K. Wang, L. P. Song and M. L. Zhang, J. Power Sources, 2009, 194, 1202–1207 CrossRef CAS.
- M. Antonietti, J. Conrad and A. F. Thünemann, Macromolecules, 1994, 27, 6007–6011 CrossRef CAS.
- M. Kasha, Molecular Excitons in Small Aggregates, Plenium Press, New York, 1976 Search PubMed.
- Y. Shen, B. Xia, A. Xie and Y. Tang, Colloids Surf., A, 2005, 252, 21–25 CrossRef CAS.
- A. Chowdhury, L. Yu, I. Raheem, L. Peteanu, L. A. Liu and D. J. Yaron, J. Phys. Chem. A, 2003, 107, 3351–3362 CrossRef CAS.
- S. M. Zhu, H. S. Zhou, M. Hibino, I. Honma and M. Ichinara, Adv. Funct. Mater., 2005, 15, 381–386 CrossRef CAS.
- R. K. Sharma, H. S. Oh, Y. G. Shul and H. Kim, Phys. B, 2008, 403, 1763–1769 CrossRef CAS.
- Y. C. Qiu, Y. H. Zhao, X. W. Yang, W. F. Li, Z. H. Wei, J. W. Xiao, S. F. Leung, Q. F. Lin, H. K. Wu, Y. G. Zhang, Z. Y. Fan and S. H. Yang, Nanoscale, 2014, 6, 3626–3631 RSC.
- A. Burke, J. Power Sources, 2000, 91, 37–50 CrossRef CAS.
- Z. Gui, H. L. Zhu and E. Gillette, ACS Nano, 2013, 7, 6037–6046 CrossRef CAS PubMed.
- C. W. Huang, C. A. Wu and S. S. Hou, Adv. Funct. Mater., 2012, 22, 4677–4685 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |