A sensitive electrochemiluminescent immunosensor based on 3D-flower-like MoS2 microspheres and using AuPt nanoparticles for signal amplification
Xiu Wanga,
Lei Shena,
Wenping Denga,
Mei Yan*a,
Haiyun Liua,
Shenguang Geb,
Jinghua Yu a and
Xianrang Songc
a and
Xianrang Songc
aSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, P. R. China. E-mail: chm_yanm@126.com; Fax: +86-531-88959988; Tel: +86-531-82767161
bShandong Provincial Key Laboratory of Preparation and Measurement of Building Materials, University of Jinan, Jinan 250022, P. R. China
cShandong Provincial Key Laboratory of Radiation Oncology, Shandong Cancer Hospital and Institute, Jinan 250117, China
First published on 25th February 2016
Abstract
We herein report the synthesis of MoS2, consisting of three-dimensional flower-like microspheres assembled by bent flakes with a thickness of several nanometers. The diameter of theses spheres was approximately 1.5 μm, and they showed good catalysis of H2O2 and could increase the electrochemiluminescence (ECL) intensity of luminol. The ECL properties of luminol were investigated and a type of luminol composite (MoS2–luminol) was prepared. Glucose oxidase–AuPt nanoparticles (GOx–AuPt) showed excellent catalytic performance for the reduction reaction of glucose. The prepared GOx–AuPt and MoS2–luminol were applied in a sandwich-type ECL immunosensor for prostate-specific antigen (PSA). In the immunosensor, MoS2–luminol acted as a solid support for PSA primary antibody, and GOx–AuPt was employed as a support for PSA secondary antibody. With the addition of glucose, hydrogen peroxide was prepared and the ECL properties of the MoS2–luminol were enhanced. The proposed immunosensor enabled PSA concentrations to be determined in the range of 0.001 ng mL−1 to 100 ng mL−1, with a detection limit of 0.28 pg mL−1. The experimental results indicated that the immunosensor exhibited simple instrumentation, high sensitivity, wide linear range and excellent analytical performance, and could be a promising technique for tumor marker detection.
1 Introduction
Transition metal chalcogenide semiconductors display interesting properties that are important for applications such as optoelectronics,1 catalysis,2 electrode materials,3,4 electrochemical hydrogen storage5 and lithium battery materials.6 As one of these, MoS2 has gained wide attention in recent years due to analogies with graphene as a potential support material for immobilized enzymes. More recently, considerable efforts have been devoted to the preparation of various MoS2-based nanomaterials such as MoS2/C nanocomposites,7,8 MoS2–graphene composites,9 MoS2–polypyrrole10 and sphere-like MoS2 nanostructures11 as the competitive electrode materials for super capacitor applications.Recently, 3D-flower-like MoS2 microspheres were successfully synthesized by a simple hydrothermal process with the help of a surfactant. The 3D-flower-like MoS2 microspheres are prepared via one step hydrothermal process, which is not only useful in synthesis of monodispersed and highly homogeneous nanoparticles, but also is considered as an environment-friendly method.12–14 The 3D-flower MoS2 microspheres had uniform sizes with diameter of about 1–2 μm and were constructed with many irregular nanosheets as a petal-like structure with thickness of several nanometers.15 A possible formation mechanism of the MoS2 microspheres was preliminarily proposed on the basis of observations of a time-dependent morphology evolution process.16 Its electrocatalytical performance is also rarely studied except for hydrodesulfurization reaction and hydrogen evolution reaction.17,18 MoS2 microspheres have showed highly electrocatalytic activity toward reduction of H2O2 which achieves highly efficiency and sensitive detection of H2O2 at the nanomolar level.19 Predictably, the success of MoS2 in these fields opens up new prospects for technological breakthroughs and encourages the exploration of MoS2 for new research fields,20 for example, biology including biomedicine, biosensors, cell-targeted labeling,21 and other fields of biology.22
AuPt nanoparticles have large specific surface area, good stability, interesting electrical, optical properties23 and biocompatibility.24,25 More importantly, AuPt nanoparticles provided a good pathway of electron transfer and enhanced the immobilized amount of biomolecules.26 Hereon, the AuPt nanoparticles can immobilize on the surface of GOx through an electrostatic incorporation decorating, the composites had a good stability.
Recently, ECL-based enzymes detection has played a significant role in the forefront of the bioanalysis area because of its ultrahigh sensitivity and selectivity.27 Furthermore, the ECL strategy can decrease the detection limit to the single-molecular level. Because enzymes exhibit outstanding specificity, selectivity, and catalytic activity, these merits have led to their widespread applications in research, medicine, and industry.28 To date, enzymes have been widely applied as recognition and signaling elements for the detection of some specific molecular analytes.29,30 Glucose oxidase (GOx) can catalyze the oxidation of glucose to gluconolactone along with the generation of hydrogen peroxide (H2O2).31 By quantifying the amount of H2O2 generated, one can indirectly quantify the amount of GOx. Here, we observed that H2O2 can efficiently increase the ECL of the intensity of luminol. Therefore, an ECL immunosensor based on the GOx system is available.
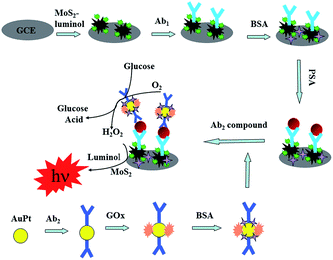
In this paper, we report a novel biosensor which employed flower-like MoS2 microspheres for embedding luminol to fabricate a sensitive ECL immunosensors for detection PSA. The prepared MoS2 microspheres were assembled by lots of bent flakes, among which also have plenty of inter spaces beneficial to adsorb lots of luminol and PSA primary antibody (Ab1) and it can increase the ECL intensity greatly. PSA secondary antibody (Ab2) was incubated on the surface of the GOx–AuPt nanoparticles. Thus, we obtained the ECL intensities, and thus the amount of PSA could be ascertained. Hence, the three-dimensional flower-like MoS2 microspheres have a very promising in the pharmaceutical, clinical and industrial application.
2 Experiments
2.1 Materials
Hydrogen tetrachloroaurate(III) hydrate (HAuCl4·3H2O, 99%) and hydrogen hexachloroplatinate(IV) hydrate (H2PtCl6, 99%) were purchased from Kojima. Silver nitrate (AgNO3, 99.8%) was purchased from Junsei. Sodium iodide (NaI, 99.5%) and L-ascorbic acid (C6H8O6, 99.5%) were supplied by Sigma-Aldrich. Hydrochloric acid (HCl, 35%) was purchased from Samchun. Cetyltrimethylammonium bromide (CTAB, C19H42BrN, 95%) and N,N′-carbonyl diimidazole (CDI) were purchased from Fluka. All chemical materials were dissolved in deionized water (18.2 MΩ). This deionized water was prepared by a Milli-Q water purification system from Millipore. Phosphate buffered solutions were prepared using 0.01 M KH2PO4 and 0.01 M Na2HPO4. All reagents were of analytical-reagent grade or the highest purity available and directly used for the following experiments without further purification. Prostate-specific antigen (PSA), the primary anti-PSA (Ab1) and the secondary anti-PSA (Ab2), bovine serum albumin (BSA, 96–99%) were gotten from Shanghai Linc-BioScience Co. Ltd. (Shanghai, China). The PSA was stored at 4 °C, and its standard solution was prepared daily with PBS solution in use. The clinical serum samples were provided by Shandong Tumor Hospital.2.2 Apparatus
UV-vis spectrum was recorded on a UV-2250 spectrophotometer (Shimadzu, Japan). Scanning electron microscope (SEM) images were recorded using a JEOL-JSM-6300 scanning electron microscope. Transmission electron microscopy (TEM) images were obtained using a Hitachi H-800 microscope (Japan). The ECL measurements were carried out using a MPI-E multifunctional electrochemical and chemiluminescent analytical system (Xi'an Remex Analytical Instrument Ltd., Co.) with the voltage of the photomultiplier tube (PMT) set at −800 V. Electrochemical measurements were performed with a CHI 760D electrochemical workstation (Shanghai CH Instruments Inc., China). Electrochemical impedance spectroscopy (EIS) was carried out on an IM6x electrochemical station (Zahner, Germany). X-ray diffraction (XRD) patterns were obtained using a D8 Advance (Bruker) X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). A conventional three electrode system was used for all electrochemical measurements: a modified glassy carbon electrode (GCE, rounded, 3 mm in diameter) as the working electrode (WE) and a Pt electrode as counter electrode (CE) and Ag/AgCl electrode as the reference electrode (RE), respectively.2.3 Preparation of 3D-flower MoS2 microspheres
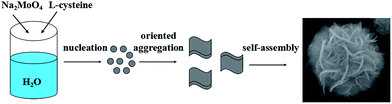
The 3D-flower MoS2 microspheres were synthesized by a one-step reaction referring to literature method.14 In brief, 0.242 g of Na2MoO4·2H2O (1.0 mmol). After ultrasonication for 5 min, the solution was adjusted to pH 6.5 with 0.1 M HCl. Then, 0.25 g of L-cysteine and 50 mL of deionized water were added to the solution followed by ultrasonication for 10 min. The mixture was then transferred into a 50 mL Teflon-lined stainless steel autoclave and reacted at 200 °C for 24 h. After that, the black precipitate was collected by centrifugation, washed three times with deionized water and ethanol, and then dried in an oven at 80 °C for 12 h.2.4 Preparation of MoS2–luminol
Preparation of MoS2–luminol, the prepared MoS2 was dipped in 5 mL of CDI solution (1%) at room temperature for 4 h with greatly magnetic stirring. Subsequently, the precipitate was collected by centrifugation. The mixture was then dispersed into 5 mL of luminol solution (1.00 × 10−4 mol L−1) and kept undisturbed under ambient conditions for 4 h. Finally, the precipitate was collected by centrifugation with deionized water for several times. 1.0 mg of amorphous MoS2–luminol and 80 μL Nafion solution (5 wt%) were dispersed in 1 mL of a solution composed of 200 μL ethanol and 800 μL deionized water.2.5 Preparation of AuPt nanoparticles aqueous solution
Gold nanoplates were prepared from 5 nm sphere seed by a three-step seed mediated method with iodide ions which was reported previously.23,24 Briefly, 0.5 mL of a 20 mM aqueous HAuCl4·3H2O solution and 1 mL of a 10 mM aqueous solution of sodium citrate and 1 mL of a 100 mM aqueous NaBH4 (ice-cold) solution were added to 36.5 mL of deionized water with vigorous stirring. In order to prepare triangular nanoplates, three labeled flasks were prepared. A mixture of 108 mL of 0.05 M aqueous CTAB solution and 54 μL of 0.1 M aqueous NaI solution was divided into three containers labeled with 1, 2, and 3. 9.0 mL of mixture was added in each container 1 and 2. The remaining mixture (90 mL) was added in container 3. Then, a mixture of 125 μL of a 20 mM aqueous HAuCl4·3H2O solution, 50 μL of 100 mM NaOH, and 50 μL of 100 mM ascorbic acid was added to each container 1 and 2. A mixture of 1.25 mL of 20 mM HAuCl4·3H2O, 0.5 mL of 100 mM NaOH, and 0.5 mL of 100 mM ascorbic acid was added to container 3. 1.0 mL of the seed solution was added to container 1 with mild shaking, followed by adding 1 mL of container 1 solution into container 2. After gentle shaking, the whole solution of container 2 was added to container 3. From that we can get gold nanoplates.In the presence of iodide ions (50 μM), 20 mL of 0.05 M CTAB, 5 mL of redispersed Au nanoplates, 30 μL of 2 mM aqueous AgNO3 solution, and 480 μL of 0.1 M aqueous ascorbic acid solution were added to a vial. The mixture was kept at 70 °C. After 1 h, 480 μL of 0.1 M HCl and 300 μL of 2 mM aqueous H2PtCl6 solution were added to the mixture with gentle shaking. The mixture was kept at 70 °C for approximately 4 h. After this reaction, the sample was centrifuged, and the supernatant was removed and redispersed in deionized water. After washing twice, AuPt nanoparticles were dispersed into 15 mL of deionized water to get the concentration was 0.1 mg mL−1 for preparation of stock solution.
2.6 Preparation of AuPt–GOx labeled Ab2
To generate AuPt nanoparticles immunological labels, 1 mL of the above AuPt suspension was mixed with 1 mL of Ab2 solution (Ab2, 20 μg mL−1, in 0.01 mol L−1 PBS) (pH 7.4). After incubation at 4 °C for 5 h, the residual antibody was removed by centrifugation and washing with 0.1 mol L−1 PBS several times. After that, the other anchor points for GOx adsorption onto AuPt were created, 2 mL of GOx (1%, in pH 7.4 PBS) was added to the solution and incubated overnight. And then, the AuPt–GOx labeled Ab2 was redispersed in 2 mL of 1% BSA solution for 2 h, under stirring, to block the excess amino groups and nonspecific binding sites of the AuPt nanoparticles labeled Ab2. After being centrifuged and washed with PBS, the resultant AuPt nanoparticles labeled Ab2 was dispersed with 0.01 mol L−1 of pH 7.4 PBS to a final volume of 2 mL, the concentration of the AuPt–GOx labeled Ab2 was 0.02 mg mL−1, and stored at 4 °C for later usage.2.7 Fabrication of the sandwich-type electrochemiluminescent immunosensor
The whole process for constructing the modified electrode was shown schematically in Scheme 1. A GCE with 3 mm diameter was polished on fine abrasive paper carefully, then with 1.0, 0.3 and 0.05 mm alumina powder was polished on chammy, and washed ultrasonically with deionized water. The prepared MoS2–luminol was dipped in 5 mL of EDS–NHS solution (1%) at room temperature for 5 h. First, 5 μL MoS2–luminol and EDS–NHS hybrids was coated on the working electrodes and dried at room temperature. Then, 5 μL of Ab1 (0.1 mol L−1 PBS, pH 7.4) was applied to the corresponding modified GCE and reacted at room temperature for 30 min. After that, excess antibodies were washed with pH 7.4 PBS and incubated in 1 wt% BSA for 1 h to block non-specific binding sites. Subsequently, the electrode was incubated with various concentrations of PSA solution. Finally, the prepared AuPt–GOx labeled Ab2 was dropped on to the electrode surface, followed by washing, and used for the PSA detection.2.8 ECL detection of PSA with the immunosensor
ECL detection of PSA with the immunosensor ECL measurements was done at room temperature. The modified GCE was dipped in the 0.01 mol L−1 PBS containing 1% glucose, and after a reaction of 8 min, the ECL intensity was measured. The potential swept from −0.2 to 0.65 V with a scan rate of 100 mV s−1 with a photomultiplier tube voltage of −800 V. The ECL signals related to the PSA concentrations could then be measured.3 Results and discussion
3.1 Characterization of 3D-flower like MoS2 microspheres
Fig. 1 shows the typical SEM images and TEM image of MoS2 sample and Scheme 2 shows schematic illustration of the MoS2 flower-like microspheres self-assembled by bent flakes. As shown in Fig. 1A and B, it displays obviously that MoS2 sample is three-dimensional flower-like microspheres assembled by bent flakes with thickness of about several nanometers, and the diameter of these spheres is approximate 1.5 μm. Moreover, there are many pores with different diameters consisting of 2D nanosheets on the surface of the MoS2 hierarchical structures and this is in favor of adsorbing small molecules. The possible formation mechanism of the self-assembled nanosheets of MoS2 flower-like microspheres prepared is illustrated in Scheme 1.14,15 In the first stage, Na2MoO4 and L-cysteine react with each other to nucleate and grow into MoS2 nanoparticles. Then MoS2 nanoparticles are oriented growth with two-dimensional direction and self-assembly form MoS2 microsphere. Fig. 1C showed a typical TEM image of MoS2 microsphere, which reveals that the as-prepared MoS2 microspheres consist of many MoS2 nanosheets. The highly wrinkled surface and extruded lamella-like structure of the microspheres could be obviously observed, which indicates that the flower-like microspheres are composed of MoS2 nanosheets. The EDS spectrum of the MoS2 microspheres (Fig. 1D) gives the signals of only element Mo and S, besides Al, no other element was observed, which is close to the stoichiometric ratio of MoS2. | ||
| Fig. 1 (A) SEM of MoS2 microspheres, (B) SEM of single MoS2 microspheres, (C) TEM of MoS2 microspheres, (D) EDS of MoS2 microspheres (point 1 on (A)). | ||
Fig. 2 showed the XRD patterns of as-prepared MoS2 synthesized by the surfactant-assisted hydrothermal method. The XRD pattern of the as-prepared MoS2 product in Fig. 2 shows diffraction peaks at 14.20°, 33.80°, 40.41° and 59.19°, which corresponds to crystal indexes of (002), (100), (103) and (110), respectively. All labeled diffraction peaks in Fig. 2 can be indexed to those of the pure hexagonal phase of MoS2 microspheres. No peaks from other impurities are detected in the XRD pattern, indicating that the sample was highly crystalline. Recently, the formation mechanism of novel flower-like nanostructures has been widely reported and discussed.32,33 Some described the fabrication of ZnO flower-like multi-sheets via a vapor–liquid–solid process.34 They indicated that self-assembly growth and Ostwald ripening mechanism may account for the growth process of ZnO flowers.35 In this study, we believe that the growing process of flower-like MoS2 microspheres is consistent with previous reports,36 the sheet-like MoS2 nanostructures gradually evolved to flower like MoS2 micro-spheres through the self-assembled process.
3.2 Characterization of AuPt–GOx composite
Fig. 3 shows SEM images and EDS of the AuPt nanostructures. A monodisperse sample of AuPt nanoparticles is a critical starting point for the synthesis of high-quality nanospheres. From the SEM images (Fig. 3A), the inset was AuPt nanostructures size distribution image, AuPt nanoparticles were observed and the diameters of the AuPt nanoparticles were about 70–80 nm. Fig. 3B was the TEM of gold nanoplates. The composition of a single particle (randomly chosen) was analyzed by EDS. The EDS spectrum of point 1 was shown in Fig. 3C.To monitor the formation of AuPt–GOx composites, we studied the UV-vis absorption spectra of the Au nanoplates, AuPt nanoparticles, GOx and AuPt–GOx composites, respectively (Fig. 3D). The Au nanoplates showed absorption peaks at 523 nm (curve a), whereas there was a peak at 554 nm for the AuPt nanoparticles (curve b). The pure GOx showed the absorptions at 373 nm and 457 nm (curve c). When GOx were encapsulated into the AuPt nanoparticles, three absorption peaks were simultaneously observed (curve d). The peaks at 380 and 441 nm were mainly ascribed to the GOx, whereas the peak at 553 nm was attributed to the AuPt nanoparticles. Compared with those obtained with pure GOx solution and pure AuPt nanoparticles, the slight deviation between absorption wave numbers was due to the interaction between AuPt nanoparticles and the GOx solution. Thus, we could conclude that the AuPt–GOx composites were prepared.
The load of GOx was an important fact of the AuPt nanoparticles. The detection process of the average amount of GOx on nanotags was as follows: first, preparation of 8 mL 0.02 mg mL−1 of GOx. Second, absorbing 4 mL 0.02 mg mL−1 of AuPt nanoparticles into a centrifuge tube, then adding 4 mL 0.02 mg mL−1 of GOx, after this reaction, the sample was centrifuged, and removed the supernatant. Third, we detect the absorbance of GOx and the supernatant at 457 nm. Finally, we can get the average amount of GOx on nanotags was 0.7 mg mg−1.
3.3 Possible mechanisms for ECL production
The MoS2–luminol/GCE showed a cathodic current signal in air-saturated pH 7.2 PBS (containing 1% glucose). In order to obtain higher sensitivity, MoS2 was used to enhance the current by reducing the barrier of electrons to MoS2. According to the literature,37–40 the reaction processed in air-saturated solution could be expressed as follows: (1) the GOx-biocatalyzed oxidization toward the added glucose led to the formation of gluconic acid and H2O2 with the participation of O2; (2) with the presence of H2O2, MoS2 microspheres were oxidized to MoS2HO˙; (3) HOO˙ produced by the reaction of H2O2 and HO˙; (4) the luminol on the surface of EGN–AuNPs nanoparticles were excited by electron (e−), forming the excited state of luminol (luminol*); (5) HOO˙ could transfer the energy of luminol* (a), generating stable-state luminol; (6) the remaining luminol* (b) could emit light (hν). The possible mechanisms of ECL are described as the following equations:| Glucose + O2 → gluconic acid + H2O2 | (1) |
| H2O2 + MoS2 → HO˙ + MoS2HO˙ | (2) |
| H2O2 + HO˙ → HOO˙ + H2O | (3) |
| Luminol + e− → luminol* | (4) |
| Luminol* (a) + 2HOO˙ → luminol + 2OH˙ + O2 | (5) |
| Luminol* (b) → luminol + hν | (6) |
3.4 ECL behaviors of the immunosensors
To investigate MoS2 nanoparticles can increase the reaction time between the as prepared immunosensor and the glucose for ECL analysis, we have an experiment to compare the enrichment features of the capture probe based on MoS2 nanosheet and 3D flower like MoS2 captures, from the Fig. 4 we can see the 3D flower MoS2 capture probe have a higher ECL intensity. We also designed another experiment, which was designed without the MoS2 nanoparticles for the sensing platform. ECL detection was done at room temperature, the modified electrode was dipped in the 0.01 mol L−1 PBS containing 1% glucose. In Fig. 5A, we compared the reaction time of MoS2–luminol composite labeled Ab1 and pure luminol labeled Ab1. As can be seen, ECL intensity of the MoS2–luminol composite labeled Ab1 increases with increasing of incubation time (a), and inclines to a constant value after 8 minutes. But the ECL intensity of the luminol labeled Ab1 increases with increasing of incubation time (b), and inclines to a constant value after 12 minutes. Fig. 5B showed the ECL profiles of the immunosensor at 8 min with MoS2 microspheres (a) and at 12 min without MoS2 microspheres (b), which can indicated that MoS2 microspheres can greatly increase the ECL intensity. As a result MoS2 composite could increase the reaction time, and also can increase the ECL signal which could greatly increase the immobilization analysis. | ||
| Fig. 4 (A) SEM of MoS2 nanosheets, (B) ECL intensity with the probe of 3D flower like MoS2 (a) and MoS2 nanosheets (b). | ||
To verify the enzymatic amplification of the immunosensor, the ECL behavior of the GCE/MoS2–luminol–Ab1/PSA/AuPt–GOx–Ab2 electrode was studied by adding 1% glucose into the aforementioned PBS solution, and the ECL signal of luminol showed a sharp increase (curve a in Fig. 5C). Compared with the ECL behavior without the addition of the glucose (curve b in Fig. 5C), the intense ECL signal for the as prepared electrode originated from the synergistic effect of the H2O2, which resulted from the GOx-mediated electrooxidation of glucose in the presence of oxygen. On the whole, the multiple enzymatic significantly amplified the generated ECL photons.
To investigate the amplification technique of the AuPt–GOx composites for ECL analysis, we designed one experiment. In Fig. 5D, we compared the ECL intensity of pure GOx labeled Ab2 and AuPt–GOx composites labeled Ab2. As can be seen, AuPt–GOx composites could increase the surface area, which could benefit for the immobilization GOx.
3.5 Electrochemical impedance spectroscopy (EIS)
EIS is an effective method for probing the features of surface modified electrodes.41–43 The impedance spectra include a semicircle portion and a linear portion. The semicircle diameter at higher frequencies corresponds to the electron-transfer resistance (Ret), and the linear part at lower frequencies corresponds to the diffusion process. EIS experiments were performed in a background solution of 2.5 mmol L−1 Fe(CN)64−/3− containing 0.1 mol L−1 KCl, and the frequency range is at 0.01 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz at 5 mV. Fig. 6A shows the EIS of the GCE at different stages. It was observed that the EIS of the bare electrode displayed an almost straight line (curve a), which was characteristic of a mass diffusion limiting process. After the GCE were coated by the MoS2–luminol composite, the semiconductor film increased the impedance, thus showing a larger Ret (curve b). Then, Ab1 and BSA could all resist the electron-transfer kinetics of the redox probe at the electrode interface, resulting in the increased impedance of the electrode (curves c, d), which verified the immobilization of these substances. Similarly, PSA and AuPt–GOx–Ab2 could both resist the electron-transfer kinetics of the redox probe at the electrode interface, showed Ret increase as a result (curve e, f). All in all, the results can also indicate the success immobilization of each modification step.
000 Hz at 5 mV. Fig. 6A shows the EIS of the GCE at different stages. It was observed that the EIS of the bare electrode displayed an almost straight line (curve a), which was characteristic of a mass diffusion limiting process. After the GCE were coated by the MoS2–luminol composite, the semiconductor film increased the impedance, thus showing a larger Ret (curve b). Then, Ab1 and BSA could all resist the electron-transfer kinetics of the redox probe at the electrode interface, resulting in the increased impedance of the electrode (curves c, d), which verified the immobilization of these substances. Similarly, PSA and AuPt–GOx–Ab2 could both resist the electron-transfer kinetics of the redox probe at the electrode interface, showed Ret increase as a result (curve e, f). All in all, the results can also indicate the success immobilization of each modification step.
3.6 Optimization of experimental conditions
To achieve an optimal ECL signal, the pH value of the substrate solution was the important factor to the ECL intensity. The study of pH influence on the ECL detection was conducted in the range of 4.5–8.5. As shown in Fig. 6B, the optimal ECL response was achieved at pH 7.2. The reason was that the highly acidic or alkaline surroundings would damage the immobilized protein.The potential range was another important factor to the ECL intensity. As can be seen in Fig. 6C, besides the anodic ECL peak, it was noted that a strong cathodic ECL peak was also observed at −0.2 to 0.65 V. As a comparison, a low ECL response was obtained at −0.2 to 0.45 V. To verify the ECL peak, the optimal ECL response was achieved at −0.2 to 0.65 V.
The concentration of glucose was also an important factor to the ECL intensity. The study of glucose concentration influence on the ECL detection was conducted in the range of 0.05–1.5%. As shown in Fig. 6D, the maximum ECL response was achieved at the concentration of glucose was 1%, and then the ECL intensity was not varied in large range.
3.7 Performance of the sandwich-type ECL immunosensor for PSA detection
Under the optimal conditions, the calibration curve for the determination of PSA is shown in Fig. 7. As can be seen in Fig. 7, the ECL intensity increased linearly with the concentration of PSA over the range 0.001 ng mL−1 to 100 ng mL−1. The regression equation was IECL = 1762.11 + 570.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cPSA (cPSA/ng mL−1) and the correlation coefficient was 0.9911 (n = 6). The limit of detection for PSA was 0.28 pg mL−1. Table 1 showed the labels and detection limit of immunosensors with previous reports.42–45 The limit of detection was much lower than those for the reported competitive biosensors (Table 1). The AuPt–GOx composite as an ECL label with the amplification technique greatly increased the sensitivity and extended the detectable concentration range by 5 orders of magnitude. Compared with other methods, the immunosensor has a relatively large linear range and low detection limit. The results demonstrated that the proposed method could be used for the determination of PSA.
cPSA (cPSA/ng mL−1) and the correlation coefficient was 0.9911 (n = 6). The limit of detection for PSA was 0.28 pg mL−1. Table 1 showed the labels and detection limit of immunosensors with previous reports.42–45 The limit of detection was much lower than those for the reported competitive biosensors (Table 1). The AuPt–GOx composite as an ECL label with the amplification technique greatly increased the sensitivity and extended the detectable concentration range by 5 orders of magnitude. Compared with other methods, the immunosensor has a relatively large linear range and low detection limit. The results demonstrated that the proposed method could be used for the determination of PSA.
3.8 Specificity, stability, reproducibility of the immunosensor
In this study, to evaluate the specificity of the electrochemical immunosensor, we investigated the system with other biomarkers, e.g. Human Chorionic Gonadotropin (HCG), α-fetoprotein (AFP), cancer 125 antigen (CA125), carcinoembryonic antigen (CEA) and cancer 199 antigen (CA199). The specificity was studied by using an incubation solution containing the known PSA standards and interfering agents with various concentrations. Fig. 8A shows the experimental data in 10 ng mL−1 (as an example) PSA solutions containing various interfering substances. As seen from experimental results, no obvious difference of stripping ECL intensity was observed toward various concentrations of interfering agents in comparison with the results obtained in the presence of only PSA. Fig. 8B showed the influence of unspecific adsorption of other proteins in the serum to the immunosensor, as seen from experimental results, no obvious difference of stripping ECL intensity was observed toward various concentrations of unspecific adsorption of other proteins in comparison with the results obtained in the presence of only PSA. Moreover, the increase of the concentration of interfering agents did not lead to a significant ECL shift. So the selectivity of the as-prepared immunosensor was acceptable.The stability of this immunosensor was tested by carrying out 9 continuous cyclic scans in 0.1 mol L−1 PBS (pH 7.2). As shown in Fig. 8C, the result suggested excellent stability. In addition, when the immunosensor was dried and stored at 4 °C, the ECL response retained 94% of the initial response after a storage period of 30 days. The slow decrease in response may be related to the gradual deactivation of the immobilized antibody incorporated in the composite.
The storage stability of the immunosensors was evaluated by measuring the colorimetric response of the modified papers and the GCE/MoS2–luminol–Ab1/PSA/AuPt–GOx–Ab2 stored at room temperature (25 °C), in a refrigerator (4 °C) and in a freezer (−20 °C). Fig. 8D shows the average storage stability under three conditions over a period of days. 5 ng mL−1 PSA was used for each test and each experiment was performed in triplicate. The stability of the immunosensors stored at room temperature was 95% of the initial response in the first 20 days and then decreased to 50% in the next 40 days. A remarkable average stability of up to 95% and 94% of the initial response was retained after 60 days of storage in the refrigerator and freezer. The results demonstrate the excellent storage stability of the immunosensor.
The reproducibility and precision of the proposed immunoassay was evaluated by inter-assay and intra-assay relative standard deviation (RSD). The detections of 1 ng mL−1 of PSA on 11 different immunosensors fabricated in dependently (inter-assay) showed an RSD of 3.12%, giving an acceptable fabrication reproducibility of this immunosensor. The RSD, for 11 parallel measurements on the same one immunosensor (intra-assay) incubated with the incubation solution containing 1 ng mL−1 of PSA was 2.56%, indicating a good precision, giving an acceptable fabrication reproducibility of the immunosensors.
3.9 Application in human serum samples
The performance of the immunosensor was tested in the analysis of human serum. The serum was provided by Shandong Tumor Hospital and was analysed using in the immunosensor. The results were compared with reference to a commercialized enzyme-linked immunosorbent assay (ELISA) method and are shown in Table 2. The relative difference between the two methods indicated that there was no significant difference between the results obtained by the two methods. The standard addition method was also used to evaluate the practical application of this immunosensor. Serum samples were prepared by adding different concentration of PSA to human serum samples. The recoveries indicated that this immunosensor may hold great promise as a viable alternative method for the determination of PSA in real clinical serum samples. As a simple, easy-to-perform and rapid detection immunosensor, this method could be used for further assessment of the health status.| This method (ng mL−1) | ELISA (ng mL−1) | ||||
|---|---|---|---|---|---|
| Sample | PSA detected | Added | Found | Recoveries | PSA detected |
| 1 | 0.36 | 0.50 | 0.85 | 97.2% | 0.35 |
| 2 | 0.41 | 1.00 | 1.43 | 104.8% | 0.40 |
| 3 | 0.33 | 2.00 | 2.35 | 106.1% | 0.36 |
| 4 | 0.38 | 3.00 | 3.36 | 94.7% | 0.37 |
4 Conclusions
This article describes an ECL immunoassay for PSA measurement. The 3D-flower-like MoS2 microspheres with uniform size and shape and greatly increase the ECL intensity of luminol, with the GOx–AuPt composite as an ideal label, which has excellent labeling properties and ECL activity with amplification techniques. Although the present assay system is focused on the determination of the target antigen molecules, it can be easily extended to the detection of other antigens or biocompounds. Importantly, this approach does not require sophisticated fabrication, and it is well suited for high through put biomedical sensing and application in both clinical and biodefense areas. The success of 3D-flower-like MoS2 microspheres will open up new prospects for technological breakthroughs in many fields and encourages the exploration of MoS2 for new research fields.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (51273084, 51473067), Shandong Provincial Excellent Youth Fund (ZR2015JL019).Notes and references
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- J. A. Galvis, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 87, 1214–1222 Search PubMed.
- H. Li, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS.
- T. Y. Wang, H. C. Zhu, J. Q. Zhuo, Z. W. Zhu, P. Papakonstantinou, G. Lubarsky, J. Lin and M. X. Li, Anal. Chem., 2013, 85, 10289–10295 CrossRef CAS PubMed.
- G. Veryasov, M. Grilc, B. Likozar and A. Jesih, Catal. Commun., 2014, 46, 183–186 CrossRef CAS.
- G. S. Bang, K. W. Nam, J. Y. Kim, J. Shin, J. W. Choi and S. Y. Choi, ACS Appl. Mater. Interfaces, 2014, 6, 7084–7089 Search PubMed.
- T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Shi, L. B. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433–3440 CrossRef CAS PubMed.
- Q. Wei, H. Qi, W. Luo, D. Tseng, S. J. Ki, Z. Wan, Z. Gorocs, L. A. Bentolila, T. T. Wu and A. Ozcan, ACS Nano, 2013, 7, 9147–9155 CrossRef CAS PubMed.
- C. J. Shih, Q. H. Wang, Y. Son, Z. Jin, D. Blankschtein and M. S. Strano, ACS Nano, 2014, 8, 5790–5798 CrossRef CAS PubMed.
- H. Zeng, J. Dai, W. Yao, D. Xiao and X. Cui, Nat. Nanotechnol., 2012, 7, 490–493 CrossRef CAS PubMed.
- Z. Yin, ACS Nano, 2012, 6, 74–80 CrossRef CAS PubMed.
- U. K. Sen and S. Mitra, ACS Appl. Mater. Interfaces, 2013, 5, 1240–1247 Search PubMed.
- H. Liu, X. Su, C. Y. Duan, X. N. Dong and Z. F. Zhu, Mater. Lett., 2014, 122, 182–185 CrossRef CAS.
- T. Yang, Y. J. Chen, B. H. Qu, L. Mei, D. N. Lei, H. N. Zhang, Q. H. Li and T. H. Wang, Electrochim. Acta, 2014, 115, 165–169 CrossRef CAS.
- X. H. Zhang, H. Tang, M. Q. Xue and C. S. Li, Mater. Lett., 2014, 130, 83–86 CrossRef CAS.
- M. Wang, G. D. Li, H. Y. Xu, Y. T. Qian and J. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 1003–1008 Search PubMed.
- K. Chang, Z. W. Mei, T. Wang, Q. Kang, S. X. Ouyang and J. H. Ye, ACS Nano, 2014, 8, 7078–7087 CrossRef CAS PubMed.
- C. F. Hang, Z. Y. Wang, Z. P. Guo and X. W. Lou, ACS Appl. Mater. Interfaces, 2012, 4, 3765–3768 Search PubMed.
- L. Fei, Y. Xu, X. F. Wu, G. Chen, Y. L. Li, B. S. Li, S. G. Deng and H. M. Luo, Nanoscale, 2014, 6, 3664–3669 RSC.
- W. Y. Yin, L. Yan, J. Yu, G. Tian, L. J. Zhou, X. P. Zheng, X. Zhang and Y. Yong, ACS Nano, 2014, 8, 6922–6933 CrossRef CAS PubMed.
- W. C. Peng, X. Wang and X. Y. Li, Nanoscale, 2014, 6, 8311–8317 RSC.
- J. Luo, P. N. Njoki, Y. Lin, D. Mott, L. Y. Wang and C. J. Zhong, Langmuir, 2006, 22, 2892–2898 CrossRef CAS PubMed.
- J. B. Xu, T. S. Zhao, Z. X. Liang and L. D. Zhu, Chem. Mater., 2008, 20, 1688–1690 CrossRef CAS.
- H. J. Jang, S. Y. Ham, J. A. I. Acapulco, Y. Y. Song, S. Hong, K. L. Shuford and S. Park, J. Am. Chem. Soc., 2014, 136, 17674–17680 CrossRef CAS PubMed.
- N. Moghimi, M. Mohapatra and K. Tong, Anal. Chem., 2015, 87, 5546–5552 CrossRef CAS PubMed.
- A. Dutta and J. Y. Ouyang, ACS Catal., 2015, 5, 1371–1380 CrossRef CAS.
- K. Kadimisetty, S. Malla, N. P. Sardesai, A. A. Joshi, R. C. Faria, N. H. Lee and J. F. Rusling, Anal. Chem., 2015, 87, 4472–4478 CrossRef CAS PubMed.
- W. Q. Lai, J. Y. Zhuang and D. P. Tang, J. Agric. Food Chem., 2015, 63, 1982–1989 CrossRef CAS PubMed.
- S. Lilienthal, Z. Shpilt, F. Wang, R. Orbach and I. Willner, ACS Appl. Mater. Interfaces, 2015, 7, 8923–8931 Search PubMed.
- X. J. Zheng, Q. Zhu, H. Q. Song, X. R. Zhao, T. Yi, H. L. Chen and X. G. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 3480–3491 Search PubMed.
- H. Su, D. D. Liu, M. Zhao, W. L. Hu, S. S. Xue and Q. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 8233–8242 Search PubMed.
- S. S. Acharyya, S. Ghosh and R. Bal, Chem. Commun., 2015, 51, 5998–6001 RSC.
- X. J. Liu, J. C. Zhao, Y. J. Cao, W. Y. Li, Y. G. Sun, J. Lu, Y. Men and J. Q. Hu, RSC Adv., 2015, 5, 47506–47510 RSC.
- N. Kumar, H. Mittal, L. Reddy, P. Nair and J. Catherine, RSC Adv., 2015, 5, 38801–38809 RSC.
- R. Udayabhaskar, B. Karthikeyan, P. Sreekanth and R. Philip, RSC Adv., 2015, 5, 13590–13597 RSC.
- J. F. Tang, H. H. Su, Y. M. Lu and S. Y. Chu, CrystEngComm, 2015, 17, 592–597 RSC.
- T. Y. Wang, H. C. Zhu, J. Q. Zhuo, Z. W. Zhu, P. Papakonstantinou, G. Lubarsky, J. Lin and M. X. Li, Anal. Chem., 2013, 85, 10289–10295 CrossRef CAS PubMed.
- J. Li, A. Staykov, T. Ishihara and K. Z. Yoshizawa, J. Chem. Phys. C, 2011, 115, 7392–7398 CrossRef CAS.
- T. Y. Wang, H. C. Zhu, J. Q. Zhuo, Z. W. Zhu, P. Papakonstantinou, G. Lubarsky, J. Lin and M. X. Li, Anal. Chem., 2013, 85, 10289–10295 CrossRef CAS PubMed.
- T. R. Lin, L. S. Zhong, L. Q. Guo, F. F. Fu and G. N. Chen, Nanoscale, 2014, 6, 11856–11862 RSC.
- S. M. Park and J. S. Yoo, Anal. Chem., 2003, 75, 455A–461A CAS.
- H. Chen, J. H. Jiang, Y. Huang, T. Deng, J. S. Li, G. L. Shen and R. Q. Yu, Sens. Actuators, B, 2006, 117, 211–218 CrossRef CAS.
- M. Prodromidis, Electrochim. Acta, 2010, 55, 4227–4233 CrossRef CAS.
- R. M. Gonzalez, S. L. Seurynck, S. A. Crowley, M. Brown, G. S. Omenn, D. F. Hayes and R. C. Zangar, J. Proteome Res., 2008, 7, 2406–2414 CrossRef CAS PubMed.
- N. Gull, P. Sen, R. H. Khan and K. Din, Langmuir, 2009, 25, 11686–11691 CrossRef CAS PubMed.
- N. Zhao, Y. Q. He, X. Mao, Y. H. Sun, X. B. Zhang, C. Z. Li, Y. H. Lin and G. D. Liu, Anal. Chem., 2014, 86, 1372–1379 CrossRef PubMed.
- S. J. Xu, Y. Liu, T. H. Wang and J. H. Li, Anal. Chem., 2015, 87, 6510–6515 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |