Nano-silver enhanced luminescence of Er3+ ions embedded in tellurite glass, vitro-ceramic and ceramic: impact of heat treatment
Abstract
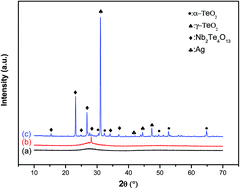
Tellurite glasses co-activated with erbium ions and silver nanoparticles (Ag NPs) are prepared using melt quenching technique. The effect of heat treatment on structural, luminescence and spectroscopic properties has been investigated. Both X-ray diffraction and Raman spectroscopy indicate the precipitation of crystalline phases after heat treatment. Based on the absorption measurements, the intensity parameters Ωt (t = 2, 4 and 6), the radiative transition probability (AT), fluorescence branching ratio (βJJ′) and radiative lifetimes (τr) of Er3+ ions were calculated using Judd–Ofelt theory. The results suggest that Er3+ ions have been incorporated into Ag NPs, which intensified the electromagnetic field around Er3+ ions. The luminescence properties of Er3+, doped prepared, were investigated. It was found that the presence of silver NPs, nucleated and grown during the heat annealing process, improves the photoluminescence (PL) intensity and the PL lifetime relative to the 4I13/2 → 4I15/2 transition. Such enhancements are attributed to the strong local electric field induced by the SPR of silver NPs as the major factor and the energy transfer from the surface of silver NPs to Er3+ ions. Using the Mc-Cumber method, emission cross-section (σe), absorption cross-section (σa), and gain cross-section (G(λ)) for the 4I13/2 → 4I15/2 transition were calculated. Compared with other glass hosts, the results indicate that the vitro-ceramic sample has good prospects as a gain medium applied for a 1.53 μm broad band and high-gain erbium-doped fiber amplifiers (EDFA).

 Please wait while we load your content...
Please wait while we load your content...