Superhydrophobic Au/polymer nanocomposite films via AACVD/swell encapsulation tandem synthesis procedure†
Sebastian C. Dixon,
William J. Peveler,
Nuruzzaman Noor,
Joseph C. Bear and
Ivan P. Parkin*
Department of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: i.p.parkin@ucl.ac.uk; Fax: +44 (0)20 7679 7463; Tel: +44 (0)20 7679 4669
First published on 21st March 2016
Abstract
A synthetic route is presented for creating well-attached Au/polymer nanocomposite thin films on glass which exhibit superhydrophobicity. Such films have been demonstrably difficult to synthesise by established means. The synthetic route devised here affords great control over the functional, physical and chemical properties of the end product. A superhydrophobic PDMS thin film is deposited on a glass substrate by aerosol-assisted chemical vapour deposition (AACVD), then gold nanoparticles are incorporated by swelling the polymer film in a dispersion of the nanoparticles in toluene, which diffuse into the polymer and become embedded upon drying. Characterisation of the nanoparticles and resultant composite films are carried out using electron microscopy (SEM and TEM), UV-visible spectroscopy, X-ray photoelectron spectroscopy (XPS) and water droplet contact angle measurements.
1. Introduction
Over the past decade, much research has investigated metal nanoparticles, on account of their high specific surface area, flexibility of application and their unique optical and catalytic properties.1–6 Materials whose utility stems from physical or chemical processes at the surface will particularly benefit from having a high specific surface area, where much of the material in the bulk would otherwise go to waste – for instance, while bulk gold is generally considered chemically inert, the high specific surface area and availability of variable oxidation states at the surface of gold nanoparticles leads to catalytic,2 antimicrobial3 and ion-sensing4 functions, while their nanoscale dimensions lend interesting and useful spectroscopic effects such as optical biosensing and pigmentation.1,5,7 In particular, nanoparticles smaller than 50 nm in diameter effect minimal scattering of visible light, enabling retention of the optical transparency in polymer nanocomposite materials.8Incorporation of nanoparticles into polymer matrices has therefore become a natural and timely progression, where functions such as those described above become desirable for consumer applications.5,7 The need for a new method for nanoparticle incorporation into polymer films is highlighted by complications with previously established methods such as supercritical fluid deposition (SFD), in which nanoparticles are synthesised in situ by a wet chemical reduction of a metal salt within the polymer matrix,9 and the ‘one-pot’ aerosol-assisted chemical vapour deposition (AACVD) method, in which nanoparticles and polymer are simultaneously delivered onto a heated substrate from a vapour.10 While these techniques have demonstrated some success, they are still afflicted with issues regarding nanoparticle dispersity and chemical compatibility respectively. Another more recently reported method immobilises gold nanoparticles at the surface of poly(methyl methacrylate) (PMMA) by covalent bonding between the nanoparticles and the thiol-functionalised surface of the polymer, but requires a number of wet chemical steps and limits the nanoparticle presence exclusively to the polymer's surface.11
Another means of introducing nanoparticles into polymer matrices does so by swelling the polymer in a compatible solvent containing a dispersion of the desired nanoparticles; after a period of time, the nanoparticles diffuse into the polymer and become trapped upon drying.12,13 This method is particularly interesting for a number of reasons; firstly, this simple procedure can be carried out with great ease and at minimal cost. Secondly, this process is applicable to a range of polymer systems, due to the simple requirements that the solvent is capable of swelling the polymer, and that the nanoparticles are dispersible in the solvent. Since a given polymer can be swollen by a number of different solvents (albeit to varying degrees),14 and nanoparticles can be functionalised to render these dispersible in a wide range of chemically different solvents,10 there is flexibility in the choice of solvent/nanoparticle system for the nanocomposite preparation. Lastly, a particular advantage of this sort of procedure is that it enables isolation of the polymer and nanoparticle syntheses, such that each can be optimally prepared then brought together into a nanocomposite in a later step. This is particularly interesting given the difficulties described with nanoparticle incorporation by other means,9,10 but also facilitates the functionalisation by nanoparticles of existing commercial polymer-based products.15
The recently-established method for deposition of superhydrophobic PDMS films via AACVD has had some success with one-pot depositions of the polymer with nanoparticles in a single step, with a view to generating novel superhydrophobic nanocomposite thin films.10 However, the selection of nanoparticles was restricted by compatibility considerations between the nanoparticles, the polymer and the dispersing medium. One example of this was the uncoated Au nanoparticles, which would interfere with the noble metal-based curing mechanism of the polymer and result in a lack of film deposition. To overcome this issue, we propose to combine the AACVD deposition of the superhydrophobic PDMS films with the swelling method for incorporation of nanoparticles, in an attempt to create novel Au/PDMS superhydrophobic nanocomposite thin films.
Advantages of superhydrophobic materials include self-cleaning,16 resistance to corrosion and biofilm formation,17 water repulsion and having an exceptionally high surface area.18 These benefits could therefore be extended to complement those of the nanoparticle functions, to create multimodal superhydrophobic nanocomposite films with a range of useful properties and applications. For example, superhydrophobic films incorporating gold nanoparticles could be directly introduced to antimicrobial photodynamic therapy in biomedical applications, in which the pairing of functions could simultaneously limit bacterial adhesion,17 while promoting microbial cell destruction respectively19 to result in a double-action ‘super-antimicrobial’ film. On the other hand, the high surface area of the superhydrophobic polymer could be combined with the incorporated nanogold to promote heterogeneous oxidation catalysis.2
2. Experimental details
2.1. Characterisation techniques
Transmission Electron Microscopy was carried out on a JEOL 2100 TEM operating at 200 kV. Micrographs were analysed in ImageJ software by automated edge finding within a range of black-and-white micrographs. Scanning Electron Microscopy was carried out on a JEOL JSM-6301F Field Emission SEM at an accelerating voltage of 5 kV. UV-visible spectra were obtained using a Perkin Elmer Fourier Transform Lambda 950 UV-visible spectrometer, measuring within a wavelength range of 400 to 700 nm in transmission mode. Attenuated Total Reflectance Fourier-Transform Infra-Red spectroscopy was carried out on a Bruker Platinum ATR FTIR instrument, measuring in the frequency range 400–4000 cm−1. Water droplet contact angles were measured using an FTA-1000 B-class drop shape instrument, dispensing 5 μL water droplets and measuring droplet shapes with Laplace–Young fitting. Scotch tape testing was performed by firm application of Scotch tape to samples, followed by removal of the tape; three iterations of this procedure were performed for a single test.2.2. Aerosol-assisted chemical vapour deposition of superhydrophobic film
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (0.5 g base material, 0.05 g curing agent) and dissolved in chloroform (∼50 mL) with stirring. The flask containing the mixture was then immediately connected to the AACVD apparatus for deposition, in order to minimise premature curing. Having heated the AACVD reactor chamber to 390 °C under flow of nitrogen, a Johnson Matthey Liquifog ultrasonic humidifier was used to generate an aerosol from the precursor solution. The precursor was often added in two halves to the flask, through a funnel under positive pressure of nitrogen, in order to reduce the difficulty of generating an aerosol from this relatively large volume of liquid. Nitrogen gas was passed through the precursor flask at a flow rate of 1.0 L min−1. The silica barrier glass plates (each 145 × 45 × 5 mm) were separated by a distance of 8 mm within the chamber. The bottom of the two plates rested upon a heated carbon block, whose temperature was monitored and regulated by a thermocouple. The top plate, as measured manually with a handheld thermocouple, reached a maximum temperature of approximately 310 °C. The temperature gradient between the two plates leads to the thermophoresis phenomenon,18 with deposition of cured elastomer microparticles occurring exclusively on the top plate. The deposition process was continued until all of the precursor solution had been converted to aerosol (approximately 45 min). At this point, the heating element was turned off and the reaction chamber allowed to cool, containing the plates, for approximately 30 min to room temperature under the flow of nitrogen. The plates were then removed from the reactor chamber and handled in air.
1 ratio (0.5 g base material, 0.05 g curing agent) and dissolved in chloroform (∼50 mL) with stirring. The flask containing the mixture was then immediately connected to the AACVD apparatus for deposition, in order to minimise premature curing. Having heated the AACVD reactor chamber to 390 °C under flow of nitrogen, a Johnson Matthey Liquifog ultrasonic humidifier was used to generate an aerosol from the precursor solution. The precursor was often added in two halves to the flask, through a funnel under positive pressure of nitrogen, in order to reduce the difficulty of generating an aerosol from this relatively large volume of liquid. Nitrogen gas was passed through the precursor flask at a flow rate of 1.0 L min−1. The silica barrier glass plates (each 145 × 45 × 5 mm) were separated by a distance of 8 mm within the chamber. The bottom of the two plates rested upon a heated carbon block, whose temperature was monitored and regulated by a thermocouple. The top plate, as measured manually with a handheld thermocouple, reached a maximum temperature of approximately 310 °C. The temperature gradient between the two plates leads to the thermophoresis phenomenon,18 with deposition of cured elastomer microparticles occurring exclusively on the top plate. The deposition process was continued until all of the precursor solution had been converted to aerosol (approximately 45 min). At this point, the heating element was turned off and the reaction chamber allowed to cool, containing the plates, for approximately 30 min to room temperature under the flow of nitrogen. The plates were then removed from the reactor chamber and handled in air.2.3. Swell encapsulation of Au nanoparticles into superhydrophobic elastomer film
1-Dodecanethiol capped gold nanoparticles (5 nm) in toluene were synthesised using a modified Brust–Schiffrin method of two-phase wet chemical reduction.20 Excess 1-dodecanethiol, which was found to degrade the polymer film, was removed by first removing the toluene under reduced pressure by rotary evaporation, then by washing the remaining purple liquid with ethanol (2 × 30 mL) followed by centrifugation at 4500 rpm for 10 min. The colourless supernatant was decanted and the purple pellet of gold nanoparticles (0.02 g) was allowed to air-dry before redispersion in toluene (30 mL). The resulting dispersion was used for swell-encapsulation experiments at three approximate concentrations: the as-dispersed (0.5 g L−1) Au nanoparticles in toluene, and those diluted from this to 0.1 and 0.025 g L−1 in toluene. Sections of superhydrophobic elastomer films on glass (20 × 10 × 5 mm) as deposited via AACVD were immersed in each dispersion, as well as in a control of pure toluene, for periods of 2 h, 24 h and 48 h. After this period, the nanoparticle dispersion was drained and the glass sample transferred to a clean, dry vial and allowed to air-dry overnight in a dark cupboard to allow the polymer film to shrink, before being rinsed in toluene then distilled water to dislodge any non-encapsulated material (see Fig. 2 for scheme). | ||
| Fig. 2 Scheme of the swell-encapsulation-shrink process of incorporating nanoparticles into superhydrophobic polymer film as deposited on glass by AACVD. | ||
3. Results
Superhydrophobic gold/polymer nanocomposite films were successfully prepared in a two-stage synthesis in which a superhydrophobic Sylgard-184 elastomer film was first deposited on a barrier glass substrate via the aerosol-assisted chemical vapour deposition (AACVD) method. The coated glass was then immersed in a dispersion of gold nanoparticles and allowed to soak for a period of time to enable swelling of the polymer film and the subsequent diffusion of the nanoparticles into the polymer matrix. After being allowed to dry, the polymer shrank down to its original dimensions and the nanoparticles subsequently became embedded within. In this way, superhydrophobic Au/polymer nanocomposites have been created at varying concentrations of nanoparticle content. The nanocomposite films were mechanically robust, with water droplet contact angles unchanged by a rigorous Scotch tape test (see ESI†).3.1. Au nanoparticle synthesis
The gold nanoparticles were synthesised via a modified Brust–Schiffrin method for two-phase water–toluene wet chemical reduction of hydrogen tetrachloroaurate by sodium borohydride in the presence of 1-dodecanethiol.20 Transmission Electron Microscopy (TEM) analysis was conducted on the nanoparticles in toluene dispersion (see Fig. 3), which were predominantly spherical with isotropy factor of 0.98. Using a sample of 338 particles, the mean nanoparticle diameter was found to be 4.7 ± 1.2 nm. Gold nanoparticles of this scale are expected to undergo the surface plasmon resonance (SPR) phenomenon, which is observed in the UV-visible absorption spectra of both the nanoparticle dispersion and the Au-incorporated films.3.2. Aerosol-assisted chemical vapour deposition of superhydrophobic film
The films deposited from the PDMS elastomer (Sylgard-184) by the AACVD method were white in colour, mechanically robust and translucent. The films were resistant to elevated temperature, decomposing only under annealing at temperatures in excess of the deposition temperature (390 °C). The polymer films were also resistant to pH, with the superhydrophobic properties remaining intact after soaking samples of the films for 1 h in aqueous solutions of HCl and NaOH (pH 0 and pH 14, see ESI†). It was found that films produced from the same elastomer material by dip-coating were totally colourless and transparent, however they lacked the microstructure and superhydrophobicity of the film deposited by AACVD.The developed microstructure (see Fig. 4) lends the film its superhydrophobic properties (see Fig. 5), with water droplet contact angles frequently exceeding θ = 160°, and a maximum measured at θ = 169°. Since this microstructure is a product of the AACVD method of deposition, the dip-coated films do not exhibit superhydrophobicity, although the intrinsic chemical hydrophobicity of the elastomer still lends contact angles greater than θ = 105° for these. FTIR analysis of the deposited elastomer film revealed peaks at 2960 and 2910 cm−1 as expected, corresponding to C–H stretching modes (see ESI†).
 | ||
| Fig. 5 5 μL water droplets on superhydrophobic Sylgard-184 film deposited by AACVD with contact angle θ = 163° (a), compared with the dip-coated film with θ = 105° (b). | ||
3.3. Swell-encapsulation of nanoparticles into superhydrophobic film
 | (1) |
A segment of the solid polymer was swollen in toluene for 24 h, resulting in growth of the segment from 14 × 8 mm to 19 × 12 mm (swelling ratio = 1.36), returning to its original dimensions after air-drying overnight. Experiments using more ‘hydrophilic’ solvents such as methanol and acetone, however, exhibited very little visible swelling of this particular polymer. These observations were consistent with those of previous authors,14 who found that polydimethylsiloxane (PDMS), which is chemically analogous to Sylgard-184, would swell in chloroform, toluene, methanol and acetone in ‘swelling ratios’ of 1.39, 1.31, 1.02 and 1.06 respectively.
The polymer coatings containing the embedded Au nanoparticles were found to retain their superhydrophobicity with Cassie–Baxter wetting, with a slightly decreasing trend in water droplet contact angle as nanoparticle loading increased. Contact angles of θ = 152°, 152°, 156° and 168° were measured with Laplace–Young droplet fitting for four of the superhydrophobic polymer composites obtained from swell-encapsulation in nanoparticle dispersions with approximate concentrations 0.5 g L−1, 0.1 g L−1, 0.025 g L−1 and 0.0 g L−1 respectively – see Fig. 6.
Swelling the superhydrophobic films in the nanoparticle dispersions enabled the nanoparticles to be drawn into the swollen polymer matrix and subsequently embedded once the substrate was removed from dispersion and allowed to dry. Surface-sensitive XPS analysis confirmed the presence of Au at the surface of the films, and demonstrated the ability of the swell-encapsulation method to control sample loading, with peak areas directly correlating with initial dispersion concentrations. The sample loaded at 0.5 g L−1 had a mean Au concentration of 0.10 ± 0.04 at% with respect to carbon, while the 0.1 g L−1 sample measured 0.034 ± 0.004 at%.
In the XPS spectra shown in Fig. 7, two pairs of doublets are evident: the first appears at 84.1 and 87.8 eV, typical of the binding energies for 4f 7/2 and 5/2 spin–orbit coupled electrons respectively in Au0 metal.21 However, a second doublet is also observed at 85.8 and 89.5 eV, which can be ascribed2 to 4f 7/2 and 5/2 electrons in the oxidised state Au+. The excess of the Au+ peak over that of the Au0 is to be expected due to both the surface sensitivity of XPS, and the fact that the outer shell of Au is in contact with the capping agent and may be oxidised to charge-compensate with the thiolate character of the capping agent.22 This, combined with the high specific surface area of Au nanoparticles of this size is what gives rise to such a strong Au+ peak.
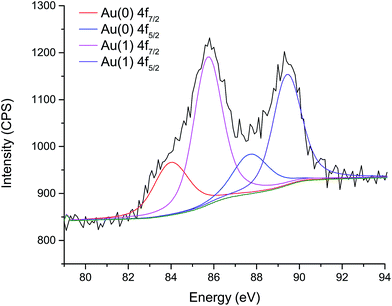 | ||
| Fig. 7 XPS spectrum in the Au 4f region of superhydrophobic PDMS film deposited by AACVD then swell-encapsulated for 2 h in a toluene dispersion of Au at approximate concentration 0.5 g L−1. The lower concentration (0.1 g L−1) XPS spectrum can be found in the ESI.† | ||
The presence of Au in the films was further supported by observation of the characteristic broad surface plasmon resonance (SPR) peak in their UV-visible absorption spectra between 470 and 700 nm, as shown in Fig. 8. The control that the swell-encapsulation technique gives over sample concentration by simple variation of the precursor concentration is supported by the relative absorbances of the SPR peaks between the 0.025 g L−1, 0.1 g L−1 and 0.5 g L−1 samples.
Since the absorbance ASPR at the SPR peak becomes increasingly diminished with decreasing particle size due to the shortening mean free path of Au surface electrons, it is generally accepted23 that diameters of Au nanoparticles of this small a scale can be determined from the ratio between ASPR and the absorbance at a suitable wavelength above and outside of the SPR absorption, A450, at λ = 450 nm. Eqn (2) can then be used to calculate nanoparticle diameter d, taking the value B1 to be the inverse of the slope 1/m of the linear fit between the ratio ASPR/A450 and ln(d/nm), and B2 = B0/m where B0 is the intercept:
 | (2) |
Taking B1 = 3.00 and B2 = 2.20 from the literature,23 the mean nanoparticle diameter can be calculated from the experimental data: taking the peak maximum at λ = 529 nm for the toluene dispersion, ASPR = 1.18 and A450 = 0.914, the mean nanoparticle diameter is therefore estimated to be 5.3 nm. This calculated figure is well-supported by the TEM sizing. Small discrepancies between diameter measurements obtained spectroscopically and microscopically do, as expected, arise on account of the effect of the nanoparticle medium and capping agent on the SPR measurement.
The SPR band of the toluene dispersion has been normalised with respect to a logarithmic fitting in order to facilitate comparison with the normalised nanocomposite spectra. A red shift in the SPR band maximum is observed for the nanoparticles incorporated within the polymer matrix from 545 nm in the normalised toluene dispersion spectrum to 575 nm for the 0.5 g L−1 2 h swell. This sort of red shift has been observed previously in Au nanocomposite thin films, and is attributed to the differences between the dielectric constants of the nanoparticles and the surrounding medium.21
Red shift has also been documented as being due to a reduction in particle spacing, which is a reasonable justification for the shift of the nanocomposite SPR peaks with respect to each other observed here, as variation in concentration of the dispersion will naturally influence the spacing of nanoparticles within the composite.21 There appears to be a red shift (15 nm) between the 0.1 g L−1 and the 0.5 g L−1 2 h swells, corresponding to a higher concentration (and therefore shorter spacing) of nanoparticles within the polymer matrix. Meanwhile, a blue shift (25 nm) is observed between the 2 h and the 48 h swells at 0.5 g L−1 concentration, possibly due to a greater degree of diffusion (and therefore a spacing-out) of nanoparticles throughout the polymer for the longer swelling time.
The relative absorbances of the SPR peaks in the films suggest that while dispersion concentration is directly related to the quantity of encapsulated material, the swelling time is not as important on these timescales. Indeed, it appears that for both the cases of 48 h swell vs. 2 h for the 0.5 g L−1 concentration, and the 24 h vs. 2 h swell at 0.1 g L−1, increased swelling time may actually be detrimental to loading quantity – this could be due to an eventual diffusion of nanoparticles out of the polymer occurring as nanoparticles begin to crash out of dispersion, thus resulting in a drop in concentration; on the other hand, and more likely when considering the stability of the Au nanoparticle dispersion in toluene, the reduced absorbance at longer swelling time may simply be due to gradual dissolution of the polymer in the organic solvent, ultimately leaving less polymer matrix available for nanoparticle encapsulation.
The most important result, however, is that we have demonstrated a method for incorporating gold nanoparticles into a superhydrophobic polymer film without loss of the superhydrophobic properties of the film (i.e. the water droplet contact angle remains greater than θ = 150°). The nanoparticles can be successfully incorporated into the polymer under mild conditions with swelling times of just 2 h in the nanoparticle dispersion. It is predicted that nanoparticles of virtually any nature, when dispersed in an appropriately selected solvent such that it swells the polymer, should be able to be incorporated into the superhydrophobic film, with the result that the presented method ought to be generic and applicable over a wide range of superhydrophobic nanocomposite systems.
4. Conclusions
It has been demonstrated that superhydrophobic Au/polymer nanocomposite thin films can be produced from a tandem AACVD–swell-encapsulation procedure. This is achieved by first generating the superhydrophobic polymer film on glass via AACVD, followed by incorporation of nanoparticles by soaking the polymer in a dispersion of Au nanoparticles in a compatible solvent then allowing to dry. The sequence presented here allows far greater control over the properties of both components of the composite material as compared with alternative methods, while it is also likely to be generally applicable to a range of nanoparticle/polymer combinations. Crucially, the superhydrophobic properties of the polymer films, as conferred by their developed microstructures, are preserved throughout the swell-encapsulation process. It has been demonstrated that control over the quantity of nanoparticles incorporated into the film is a trivial matter of simply adjusting the precursor concentration, as supported by elemental and spectroscopic analyses. It is anticipated that this work will set the foundation for future studies investigating the functional properties of these films such as antimicrobial or catalytic behaviour, as well as providing a means for a range of previously unattainable novel superhydrophobic nanocomposite materials to be synthesised.References
- S. Eustis and M. A. el-Sayed, Chem. Soc. Rev., 2006, 35(3), 209–217, 10.1039/b514191e.
- M. P. Casaletto, A. Longo, A. Martorana, A. Prestianni and A. M. Venezia, Surf. Interface Anal., 2006, 38(4), 215–218, DOI:10.1002/sia.2180.
- S. Perni, C. Piccirillo, J. Pratten, P. Prokopovich, W. Chrzanowski, I. P. Parkin and M. Wilson, Biomaterials, 2009, 30(1), 89–93, DOI:10.1016/j.biomaterials.2008.09.020.
- Y. Long, Y. Wang, Y. Liu, Q. Zeng and Y. Li, Sci. China: Chem., 2015, 58(4), 666–672, DOI:10.1007/s11426-014-5292-7.
- O. Parlak and M. M. Demir, ACS Appl. Mater. Interfaces, 2011, 3(11), 4306–4314, DOI:10.1021/am200983h.
- W. J. Peveler and I. P. Parkin, RSC Adv., 2013, 3(44), 21919, 10.1039/c3ra44842h.
- J. A. García, D. Monzón-Hernández, J. Manríquez and E. Bustos, Opt. Mater., 2016, 51, 208–212, DOI:10.1016/j.optmat.2015.11.038.
- W. Caseri, Chem. Eng. Commun., 2008, 196(5), 549–572, DOI:10.1080/00986440802483954.
- S. E. Bozbag, U. Unal, M. A. Kurykin, C. J. Ayala, M. Aindow and C. Erkey, J. Phys. Chem. C, 2013, 117(13), 6777–6787, DOI:10.1021/jp311641g.
- C. R. Crick, J. C. Bear, P. Southern and I. P. Parkin, J. Mater. Chem. A, 2013, 1(13), 4336, 10.1039/c3ta01629c.
- Y. Jin, K. H. Wong and A. M. Granville, Colloids Surf., A, 2016, 492, 100–109, DOI:10.1016/j.colsurfa.2015.11.025.
- S. Noimark, C. W. Dunnill, C. W. M. Kay, S. Perni, P. Prokopovich, S. Ismail, M. Wilson and I. P. Parkin, J. Mater. Chem., 2012, 22(30), 15388–15396, 10.1039/c2jm31987j.
- C. R. Crick, S. Noimark, W. J. Peveler, J. C. Bear, A. P. Ivanov, J. B. Edel and I. P. Parkin, RSC Adv., 2015, 5(66), 53789–53795, 10.1039/C5RA08788K.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75(23), 6544–6554, DOI:10.1021/ac0346712.
- S. Noimark, J. Weiner, N. Noor, E. Allan, C. K. Williams, M. S. P. Shaffer and I. P. Parkin, Adv. Funct. Mater., 2015, 25(9), 1367–1373, DOI:10.1002/adfm.201402980.
- P. Marchand, I. A. Hassan, I. P. Parkin and C. J. Carmalt, Dalton Trans., 2013, 42(26), 9406–9422, 10.1039/c3dt50607j.
- Z. Wang, Y. Su, Q. Li, Y. Liu, Z. She, F. Chen, L. Li, X. Zhang and P. Zhang, Mater. Charact., 2015, 99, 200–209, DOI:10.1016/j.matchar.2014.12.004.
- C. R. Crick and I. P. Parkin, Thin Solid Films, 2010, 518(15), 4328–4335, DOI:10.1016/j.tsf.2010.02.040.
- S. Perni, P. Prokopovich, J. Pratten, I. P. Parkin and M. Wilson, Photochem. Photobiol. Sci., 2011, 10(5), 712–720, 10.1039/c0pp00360c.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, Chem. Commun., 1994, 801–802, 10.1039/c39940000801.
- R. G. Palgrave and I. P. Parkin, J. Am. Chem. Soc., 2006, 128(5), 1587–1597, DOI:10.1021/ja055563v.
- R. Jin, Nanoscale, 2010, 2(3), 343–362, DOI:C10.1039/B9NR00160C.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Anal. Chem., 2007, 79(11), 4215–4221, DOI:10.1021/ac0702084.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00176a |
| This journal is © The Royal Society of Chemistry 2016 |





