A selective fluorescent probe based on bis-Schiff base for “turn-on” detection of Al3+ and cysteine by different mechanisms†
Yang Lia,
Caiyun Liaoa,
Shanshan Huanga,
Hui Xua,
Baozhan Zhenga,
Juan Du*a and
Dan Xiao*ab
aDepartment of Chemistry, Sichuan University, No. 29 Wangjiang Road, Chengdu 610064, China. E-mail: xiaodan@scu.edu.cn; lxdj@vip.sina.com
bDepartment of Chemical Engineering, Sichuan University, No. 29 Wangjiang Road, Chengdu 610064, China
First published on 2nd March 2016
Abstract
In the paper, a novel fluorescent sensor L based on phenolphthalein derivative bis-Schiff base was synthesized and characterized. Chemosensor L was found to be an excellent specific receptor for Al3+ via significant fluorescent enhancement resulting from the inhibition of an internal charge transfer (ICT) process and an efficient chelation-enhanced fluorescence (CHEF) effect. A good linear relationship was obtained in the Al3+ concentration from 0 to 50 μM with the detection limit reaching below 15 nM. Moreover, the addition of 2 equiv. of Cu2+ can almost completely quench the emission intensity of L due to a ligand–metal charge transfer (LMCT) process. The resulting L–Cu(II) complex showed a satisfactory sensing ability toward cysteine (Cys) through naked-eye and recovered fluorescence intensity with the detection limit of 0.36 nM. The fluorescence recovering process was caused by the stronger coordination ability of Cys with Cu2+ than with L and therefore free L was released from the L–Cu(II) complex. Furthermore, the sensor was used to detect Cys in practical samples with a high accuracy.
Introduction
The development of selective and sensitive chemosensors towards various metal ions has received considerable attention due to its potential applications in medicine, biochemistry and environment.1,2 Most of the metal ions play very important roles in living systems and affect human health,3,4 Gupta et al. have successfully reported selective analytical methodologies for detection of various metal ions.5–12 Among metals, aluminum is the third most abundant (approximately 8% weight) element in the earth's crust and widely found in most animal and plant tissues as well as in natural water.13 But mounting evidences suggest that excess aluminum causes damage to the central nervous system, which is suspected to be involved in idiopathic Parkinson's and Alzheimer's Disease.14,15 Therefore, it is important to design and synthesize efficient chemosensors for selective detection of Al3+ in organism and environment.As intracellular thiol-containing amino acid, cysteine (Cys) plays crucial roles for its participation in the process of biological functions.16 However, abnormal levels of Cys in human serum and urine are connected with many diseases, such as hematopoiesis decrease, hair depigmentation, skin lesions, liver damage, and fat loss.17,18 Therefore, it is of great importance to develop a simple, efficient and inexpensive probe for the recognition of Cys. Up to date, various strategies, such as high-performance liquid chromatography (HPLC), capillary electrophoresis and optical analysis have been designed to detect Cys.19–22 Among these methods, colorimetric and fluorescent probes have attracted increasing attention in recent years due to the operational simplicity, real-time detection and good sensitivity.
Recently, modified nitrogenous organic molecules have been proposed to applied as cation and anion sensor.23–27 Owing to their good photophysical properties, Schiff base derivatives equipped with a fluorescent moiety have become appealing chemosensors for the colorimetric and fluorometric determination of metal ions.28–30 Especially for bis-Schiff base molecules, due to the peculiar D–A–A–D arrangement (where, D = donor, and A = acceptor), these systems can simultaneously supply two donor sites for metal ions and therefore have stronger coordination ability than single Schiff base molecules. Although fluorescent probes for metal ions have been extensively explored, there are still only few examples of simultaneously “turn-on” and “turn-off” type probes for different metal ions and sequential “on-off-on” type sensors available for thiol-containing amino acid.31,32
In the present work, we report a new bis-Schiff base phenolphthalein derivative L as a selective fluorescent chemosensor which exhibits high selectivity, good sensitivity, low detection limit for Al3+ and Cys. Chemosensor L was synthesized in a simple reaction and characterized by 1H NMR, 13C NMR, ESI-mass and FT-IR spectrometry. The structure of chemosensor L contains two –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups acting as nitrogen–oxygen-rich coordination environments which provide enough binding sites for metallic cations. The chemosensing behaviour of L was investigated using UV-vis and fluorescence measurements. L exhibited a fluorescence enhancement behavior with 42 nm blue shift from 520 nm to 478 nm upon binding to Al3+ in ethanol. A linear response to Al3+ concentrations from 0 to 50 μM with a detection limit of 15 nM was obtained. Furthermore, the fluorescence of L was almost completely quenched by adding 2 equiv. of Cu2+ and the resulting L–Cu(II) complex triggered a green fluorescence restored and changed the solution color from yellow back to colorless again when treated with Cys. The as-prepared chemsensor exhibited a linear response to Cys concentrations ranging from 0 to 20 μM with detection limit of 0.36 nM. This method laid the foundation for the practical applications of Cys detection in physiological environments.
N– groups acting as nitrogen–oxygen-rich coordination environments which provide enough binding sites for metallic cations. The chemosensing behaviour of L was investigated using UV-vis and fluorescence measurements. L exhibited a fluorescence enhancement behavior with 42 nm blue shift from 520 nm to 478 nm upon binding to Al3+ in ethanol. A linear response to Al3+ concentrations from 0 to 50 μM with a detection limit of 15 nM was obtained. Furthermore, the fluorescence of L was almost completely quenched by adding 2 equiv. of Cu2+ and the resulting L–Cu(II) complex triggered a green fluorescence restored and changed the solution color from yellow back to colorless again when treated with Cys. The as-prepared chemsensor exhibited a linear response to Cys concentrations ranging from 0 to 20 μM with detection limit of 0.36 nM. This method laid the foundation for the practical applications of Cys detection in physiological environments.
Experimental
Reagents and apparatus
Phenolphthalein, 4-aminoantipryrine and trifluoroacetic acid (TFA) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China); hexamethylenetetramine was obtained from Chengdu Kelong Chemicals (Chengdu, China); metal ions of nitrate salts (Al3+, Mn2+, Fe3+, Co2+, Ni2+, Pb2+, Zn2+, Cd2+, Hg2+, Na+, K+, Mg2+, Ca2+, Cr3+, Ag+ and Cu2+) were purchased from Tianjin Fuyu Fine Chemical Co., Ltd. (Tianjin, China); amino acids (phenylalanine, alanine, glutamine, arginine, lysine, tyrosine, leucine, proline, serine, threonine, asparagine, valine, histidine, methionine and cysteine) were obtained from Chengdu Kelong Chemicals (Chengdu, China); glutathione was purchased from Alfa Aesar China Chemical Co., Ltd. (Shanghai, China); anhydrous ethanol was provided by Kemiou (Tianjin, China). The human serum samples were obtained from Wangjiang Hospital of Sichuan University. All the other chemicals were obtained from commercial suppliers and used without further purification, all the reagents and solvents were of analytic grade.Fluorescence measurements were acquired on F-7000 spectrophotometer equipped with a 1 cm quartz cell (HITACHI, Japan). UV-visible measurements were measured on U-2900 spectrophotometer (HITACHI, Japan). 1H-NMR and 13C-NMR were measured on a Bruker AV II-400 MHz spectrometer with chemical shifts reported in ppm (in DMSO-d6; TMS as internal standard). Mass spectra was obtained from the MAT-261 spectrometer (Thermo Fisher Scientific, San Jose, CA). FT-IR was recorded on Thermo Scientific Nicolet 6700 FT-IR spectrometer (Sugar Land, TX, USA).
Solution preparation
The sensor L stock solution (5.00 × 10−4 M) was prepared using a 100 mL volumetric flask with 37.2 mg of sensor L dissolved in ethanol and then diluted to proper concentration. Stock solutions of various metal ions (5 mM) were prepared using nitrate salts in two-distilled water. The Al3+ stock solution (2.00 × 10−2 M) was prepared in a 10 mL colorimetric tube by dissolving Al(NO3)3·9H2O (75.0 mg, 0.2 mM). The Cys stock solution (2.00 × 10−2 M) was prepared in H2O and then diluted to the mark with two-distilled water. The bis–tris buffer stock solution was prepared in two-distilled water (20 mM, pH = 7.4) with the requisite amount of HCl.In titration experiments, each time a 2 mL solution of 10 μM L was filled in microcalorimetric reaction cell of 1 cm optical path length, and then microliter solution of ions stock were added. UV absorption spectra and fluorescence spectra were taken at room temperature.
Synthesis of chemosensor L
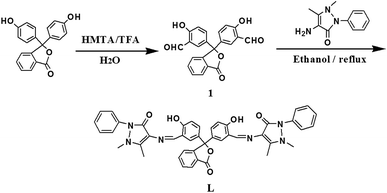
The intermediate (compound 1) was synthesized as reported procedure.33 From phenolphthalein reacted with trifluoroacetic acid to get compound 1, and then it was further condensed with 4-aminoantipryrine to give receptor L. The synthetic route was carried out as outlined in Scheme 1.An ethanol solution of 4-aminoantipryrine (40.3 mg, 2 mM) was added to a solution containing compound 1 (37.4 mg, 1 mM) in ethanol. Then the reaction mixture was stirred at 80 °C for 3 h under reflux. After cooling to room temperature, the yellow solid was filtered and washed by ethanol several time, then recrystallized with ethanol to get the final product. Yield: 68%; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 9.77 (s, 2H), 8.15–8.02 (t, J = 6.64 Hz, 2H), 7.99–7.94 (s, 1H), 7.88–7.75 (s, 1H), 7.74–7.60 (t, J = 7.73 Hz, 4H), 7.53 (m, 8H), 7.43–7.33 (dd, J = 8.67 Hz, 2H), 7.12–7.02 (d, J = 8.68 Hz, 2H), 3.51 (s, 3H), 3.32 (s, 6H), 2.64 (s, 1H), 2.50 (s, 6H) (Fig. S1†). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 169.34, 159.86, 157.01, 150.67, 134.61, 129.66, 125.42, 120.45, 117.33, 114.44, 91.03, 40.62, 40.20, 39.99, 39.79, 35.52, 10.26 (Fig. S2†). FT-IR (KBr, cm−1) ν: 3448.1, 2951.6, 1758.6, 1661.1, 1558.7, 1489.9, 1455.8, 1375.4, 1292.3, 1134.7, 1083.5, 934.0, 763.5, 696.9 (Fig. S3†). ESI-MS: calcd for C44H36N6O6 = 744.3; found [m/z] = 745.22 [M + H]+, calcd for C44H36N6NaO6 ([M + Na]+): 767.20, found: 767.20 (Fig. S4†).
Results and discussion
The metal cations response properties of L were initially studied by fluorescence analysis. The fluorogenic behavior of L was investigated with various metal cations. As shown in Fig. 1, the comparatively weak fluorescence intensity of L alone could be assigned to the intramolecular charge transfer (ICT) process. Among all the tested metal ions, only Al3+ and Cu2+ induced significant fluorescence responses of L (10 μM) upon addition of metal nitrate salts (5 equiv.). When L was treated with Al3+, a significant fluorescence enhancement with a 42 nm blue shift of emission wavelength (Fig. 1a), accompanying with a development of the fluorescence color from weak green to strong blue (Fig. 1b) can be observed. The blue shift in wavelength can be explained by the inhibition of both ICT and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation processes in Schiff base structrues,34,35 and the remarkable fluorescence enhancement was mainly attributed to the chelation of the nitrogen and oxygen atoms of L with Al3+, which resulted in efficient chelation enhanced fluorescence (CHEF) effect (Scheme 2a).36–38
N isomerisation processes in Schiff base structrues,34,35 and the remarkable fluorescence enhancement was mainly attributed to the chelation of the nitrogen and oxygen atoms of L with Al3+, which resulted in efficient chelation enhanced fluorescence (CHEF) effect (Scheme 2a).36–38
 | ||
| Scheme 2 The proposed binding mechanism of L for Al3+/Cu2+ and the proposed response mechanism of L–Cu(II) for Cys; the inset photographs were taken under a hand-held UV lamp (365 nm). | ||
More specifically, the fluorescence intensity of L at 520 nm was almost completely quenched upon addition of 2 equiv. Cu2+. The quenching behavior by Cu2+ was most likely due to a ligand–metal charge transfer (LMCT) process occurring from L to the open-shell d-orbitals of Cu2+, which motivated a faster and more efficient nonradiative decay of the excited states of L (Scheme 2b).39 Furthermore, the fluorescence of the resulting L–Cu(II) was found to be restored when treated with Cys (Fig. 2). It has been reported that the thiol–amino–carboxylic-containing amino acid can coordinate well to Cu2+.40,41 The recovery of fluorescence emission intensity indicated that the strong coordination of Cu2+ with Cys directly led to the release of free L from L–Cu(II) complex (Scheme 2c). The results clearly showed that L had a satisfactory selectivity for Al3+ and Cys by fluorescence shift/enhancement and fluorescence on-off-on process, respectively.
To further study the selectivity of L as an ion-selective colorimetric chemosensor, the absorption behavior of L was also investigated with various metal ions. The solution of L produced absorption bands centered at 285 nm and 354 nm respectively, which could be attributed to π–π* transitions of aromatic rings and n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups.42 When 1 equiv. of various metal nitrate salts was added into a 50 μM ethanol solution of L, as shown in Fig. 3, only Al3+ and Cu2+ can obviously change the absorption response of L (50 μM). A new absorption band centered at 410 nm appeared upon the addition of Al3+. It may due to the deprotonation process which leading to a stronger binding affinity of L with Al3+. Meanwhile, the original absorption band centered at 354 nm decreased and an isosbestic points at 379 nm was generated, which indicated the formation of a new complex between L and Al3+. Another significant absorption change was also observed upon the addition of Cu2+. The new absorption band centered at 420 nm and generated isosbestic points at 393 nm, clearly indicated the presence of L–Cu(II) complex between L and Cu2+. Upon addition of Cys, the absorption curve became nearly the same as that of L and the solution produced an instant color change from yellow to colorless again (insets of Fig. 3).
N groups.42 When 1 equiv. of various metal nitrate salts was added into a 50 μM ethanol solution of L, as shown in Fig. 3, only Al3+ and Cu2+ can obviously change the absorption response of L (50 μM). A new absorption band centered at 410 nm appeared upon the addition of Al3+. It may due to the deprotonation process which leading to a stronger binding affinity of L with Al3+. Meanwhile, the original absorption band centered at 354 nm decreased and an isosbestic points at 379 nm was generated, which indicated the formation of a new complex between L and Al3+. Another significant absorption change was also observed upon the addition of Cu2+. The new absorption band centered at 420 nm and generated isosbestic points at 393 nm, clearly indicated the presence of L–Cu(II) complex between L and Cu2+. Upon addition of Cys, the absorption curve became nearly the same as that of L and the solution produced an instant color change from yellow to colorless again (insets of Fig. 3).
To study the fluorescence-sensing behavior of L, quantitative investigation of the binding affinity of L with Al3+ was studied by fluorescence titration. Fig. 4 revealed an increasing fluorescence enhancement with 42 nm blue shift from 520 nm to 478 nm in the addition of 0 to 50 μM Al3+. As shown in the inset picture of Fig. 4, a good linearity (R2 = 0.9903) of the fluorescence enhancement rate F/F0 − 1 as the function of the concentration of Al3+ between 0 and 50 μM was established. The detection limit of L for Al3+ ion was calculated to be about 15 nM with a signal-to-noise ratio (S/N) of 3,43 which was about two orders lower than the WHO guideline (7.41 μM) for Al3+ in the drinking water,44 and lower than most of the reported Schiff-base chemosensors for Al3+ (Table S1†). The fluorescence quantum yields of L without and with Al3+ were 0.02 and 0.149 (versus quinine sulfate as referencer material, Φ = 0.54), respectively45 (Fig. S5†). To understand the stoichiometric nature of chemosensor L, the Job's plot for the binding activity between L and Al3+ was studied, and the result exhibited a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry from the fluorescence titration (Fig. 5A). Furthermore, the association constant (Ka) was calculated to be 2.63 × 104 M−1 according to a Benesi–Hildebrand plot46 (Fig. 5B).
2 stoichiometry from the fluorescence titration (Fig. 5A). Furthermore, the association constant (Ka) was calculated to be 2.63 × 104 M−1 according to a Benesi–Hildebrand plot46 (Fig. 5B).
The competition experiments in the same conditions were carried out for the purpose of further checking the performance of L as an Al3+-selective fluorescent sensor. When L was treated with 2 equiv. of Al3+ in the presence of other metal ions of the same concentration, the fluorescence intensities of all these mixture solutions were significantly enhanced compared with the L–Mn+ system without Al3+ (Fig. 6), except that Hg2+, Fe3+ and Ni2+ induced a relatively less degree of fluorescence increase. All of these observations showed that common coexisting metal ions did not interfere with the measurement of Al3+.
 | ||
| Fig. 6 Selectivity of the sensing system of Al3+ (2 equiv.) to other competing metal ions (50 μM) of L (10 μM) with an excitation of 375 nm. | ||
The fluorescence of L–Cu(II) was found to be restored when treated with Cys. As shown in Fig. 7a, the L–Cu(II) complex showed almost no fluorescence enhancement in response to most amino acids including methionine. It is also important to have analyte discrimination in the presence of other SH-containing compound, Fig. 7a has illustrated that most of them could not recover the fluorescence of L except Cys. A large fluorescence enhancement at 520 nm was observed, which should be caused by the release of L from the L–Cu(II) complex. In addition, it should be noticed that the thiol–amino–carboxylic-containing peptide glutathione (GSH) had comparatively obvious interference in the detection of Cys by L–Cu(II) complex. These results indicated that L–Cu2+ could be a high selectivity for Cys over competing amino acids and SH-containing compound.
Moreover, the ensuing addition of Cys to the systems containing competitive amino acids and SH-containing compound still resulted in similar fluorescence enhancement, indicating that the probe displayed a high selectivity for Cys in competitive environments (Fig. 7b).
Quantitative analysis of the in situ generated L–Cu(II) complex ensemble toward Cys was investigated by fluorescent titration. As shown in Fig. 8, 30 μM Cys can restore the fluorescence intensity of L (F0) to as much as F/F0 ∼ 96%. A linear relationship was observed between the fluorescence intensity and the concentration of Cys from 0 to 20 μM with correlation coefficient of 0.998 and the detection limit was calculated at around 0.36 nM (S/N = 3). The association constant (Kc) of L–Cu(II) complex with Cys was calculated as 2.12 × 105 M−1 according to Benesi–Hildebrand equation (Fig. S6†). We also compared our probe with other organic chemosensors (Table S2†), and the results showed that our chemsensor L provided similar linear range but much lower detection limit for the detection of Cys.
To examine the chemical reversibility of the receptor L, we conducted reversibility experiments by using EDTA for Al3+ and Cu2+ for Cys. It was found that the relative fluorescence intensity (F/F0) of L–Al3+ (10 μM) at 478 nm was distinctly reduced by the addition of 1.0 equiv. of EDTA in ethanol solution. When excessive Al3+ was added to the mixture (10 μM), the value of F/F0 increased again (Fig. 9a). The alternate additions of a constant amount of Al3+ and EDTA to the solution of L gave rise to a switchable change in the relative fluorescence intensity (F/F0). Similarly, the relative fluorescence intensity (F/F0) of L–Cu2+ (10 μM) at 520 nm was firstly recovered by the addition of 1.0 equiv. of Cys and then quenched with the addition of Cu2+ in 10% aqueous ethanol solution (Fig. 9b). The corresponding RSD values were 1.8% and 2.1%, respectively. Such a reversible fluorescence behavior of L can be repeated several times by the modulation of Al3+/EDTA and Cys/Cu2+ addition. It could be inferred that L can perform as a recycle probe for Al3+ and Cys in practical utilization.
 | ||
| Fig. 9 (a) Reversibility of L for Al3+ with EDTA in ethanol; (b) Cys with Cu2+ in 10% aqueous ethanol solution. | ||
The potential utility of L–Cu(II) complex in biological samples was also investigated, by applying the developed sensor to determine Cys in blood serum samples. Herein, 10 μL of blood serum sample was added to 50 mL of 10 μM L–Cu(II) in ethanol/bis–tris (20 mM, pH = 7.4) (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and analysis of the unknown sample was performed by the standard addition method. The Cys concentrations in blood serum samples cannot be detected by the method. Different concentrations of Cys standards were then added to the sample and the mixtures were measured. The fluorescence intensity of L with blood serum was restored following additions of different amounts of Cys. The linearly fitting the F/F0 with the concentration of added Cys was established (Fig. S7†). Recovery experiments were carried out by adding known amounts of Cys to the human serum sample. According to the analytical results shown in Table 1, the recovery of Cys was between 98% and 105% with RSD < 5%, which demonstrated that the developed sensor possessed a good potential for detecting Cys under physiological conditions.
1, v/v), and analysis of the unknown sample was performed by the standard addition method. The Cys concentrations in blood serum samples cannot be detected by the method. Different concentrations of Cys standards were then added to the sample and the mixtures were measured. The fluorescence intensity of L with blood serum was restored following additions of different amounts of Cys. The linearly fitting the F/F0 with the concentration of added Cys was established (Fig. S7†). Recovery experiments were carried out by adding known amounts of Cys to the human serum sample. According to the analytical results shown in Table 1, the recovery of Cys was between 98% and 105% with RSD < 5%, which demonstrated that the developed sensor possessed a good potential for detecting Cys under physiological conditions.
| Sample | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 5.00 | 4.93 | 98.6 | 2.5 |
| 5.00 | 5.06 | 101.2 | ||
| 5.00 | 5.18 | 103.6 | ||
| 2 | 10.00 | 10.36 | 103.6 | 2.8 |
| 10.00 | 9.89 | 98.9 | ||
| 10.00 | 10.41 | 104.1 |
Conclusions
In summary, we have designed and synthesized a novel fluorescent chemosensor L based on phenolphthalein bis-Schiff base for detection of Al3+ and amino acids Cys in buffer solution. L showed a turn-on and blue shift fluorescent response in the presence of Al3+ with the detection limit reaching below 15 nM. The addition of 2 equiv. of Cu2+ can almost completely quench the emission intensity of L, and the in situ generated L–Cu(II) complex showed a turn-on fluorescent response in the presence of Cys with the detection limit of 0.36 nM. The detection mechanisms were analyzed to be the inhibition of ICT process and CHEF effect for Al3+ detection and a ligand displacement mechanism for Cys detection, respectively. The chemosensor L could act as simultaneously “turn-on” and “turn-off” type probe for different metal ions and sequential “on-off-on” type sensors available for thiol-containing amino acid, and it exhibited high selectivity, good sensitivity, low detection limit for Al3+ and Cys. Furthermore, the chemosensor L can be used to detect Cys in blood serum samples with a high accuracy in the practical applications, suggesting its potential and significance in bioanalysis and detection in the future.Acknowledgements
The work described in this paper was supported by the National Natural Science Foundation of China (21377089, 21407109). We would like to express our sincere thanks to Analytical & Testing Centre of Sichuan University for the MS measurements.Notes and references
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS PubMed.
- J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780–3799 CrossRef CAS PubMed.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008–2012 CrossRef CAS PubMed.
- Z. Q. Guo, W. Q. Chen and X. M. Duan, Org. Lett., 2010, 12, 2202–2205 CrossRef CAS PubMed.
- V. K. Gupta, M. R. Ganjali, P. Norouzi, H. Khani, A. Nayak and S. Agarwal, Crit. Rev. Anal. Chem., 2011, 41, 282–313 CrossRef CAS.
- V. K. Gupta, A. K. Jain, G. Maheshwari, H. Lang and Z. Ishtaiwi, Sens. Actuators, B, 2006, 117, 99–106 CrossRef CAS.
- V. K. Gupta, A. K. Jain, P. Kumar, S. Agarwal and G. Maheshwari, Sens. Actuators, B, 2006, 113, 182–186 CrossRef CAS.
- S. K. Srivastava, V. K. Gupta and S. Jain, Anal. Chem., 1996, 68, 1272–1275 CrossRef CAS PubMed.
- V. K. Gupta, S. Chandra and R. Mangla, Electrochim. Acta, 2002, 47, 1579–1586 CrossRef CAS.
- V. K. Gupta, S. Jain and U. Khurana, Electroanalysis, 1997, 9, 478–480 CrossRef CAS.
- A. K. Jain, V. K. Gupta, L. P. Singh and U. Khurana, Analyst, 1997, 122, 583–586 RSC.
- V. K. Gupta, L. P. Singh, R. Singh, N. Upadhyay, S. P. Kaur and B. Sethi, J. Mol. Liq., 2012, 174, 11–16 CrossRef CAS.
- C. Y. Li, Y. Zhou, Y. F. Li, C. X. Zou and X. F. Kong, Sens. Actuators, B, 2013, 186, 360–366 CrossRef CAS.
- R. A. Yokel, Coord. Chem. Rev., 2002, 228, 97–113 CrossRef CAS.
- J. R. Cannon and J. T. Greenamyre, Toxicol. Sci., 2011, 124, 225–250 CrossRef CAS PubMed.
- H. Li, J. Liu, Y. Fang, Y. Qin, S. Xu, Y. Liu and E. Wang, Biosens. Bioelectron., 2013, 41, 563–568 CrossRef CAS PubMed.
- S. Shahrokhian, Anal. Chem., 2001, 73, 5972–5978 CrossRef CAS PubMed.
- A. Meister, J. Biol. Chem., 1988, 263, 17205–17208 CAS.
- W. Sawuła, Z. Banecka-Majkutewicz, L. Kadziński, J. Jakóbkiewicz-Banecka, G. Wegrzyn, W. Nyka and B. Banecki, Acta Biochim. Pol., 2008, 55, 119–125 Search PubMed.
- H. Yu, L. Xu and T. You, Luminescence, 2013, 28, 217–221 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341–8343 RSC.
- V. K. Gupta, S. Chandra and H. Lang, Talanta, 2005, 66, 575–580 CrossRef CAS PubMed.
- V. K. Gupta, R. Prasad and A. Kumar, Talanta, 2003, 60, 149–160 CrossRef CAS PubMed.
- V. K. Gupta, A. K. Jain and P. Kumar, Sens. Actuators, B, 2006, 120, 259–265 CrossRef CAS.
- R. Prasad, V. K. Gupta and A. Kumar, Anal. Chim. Acta, 2004, 508, 61–70 CrossRef CAS.
- V. K. Gupta, R. Prasad, P. Kumar and R. Mangla, Anal. Chim. Acta, 2000, 420, 19–27 CrossRef CAS.
- S. Padhye and G. B. Kauffman, Coord. Chem. Rev., 1985, 63, 127–160 CrossRef CAS.
- R. Azadbakht and S. Rashidi, Spectrochim. Acta, Part A, 2014, 127, 329–334 CrossRef CAS PubMed.
- V. K. Gupta, A. K. Singh, S. Mehtab and B. Gupta, Anal. Chim. Acta, 2006, 566, 5–10 CrossRef CAS.
- C. Gou, S. H. Qin, H. Q. Wu, Y. Wang, J. Luo and X. Y. Liu, Inorg. Chem. Commun., 2011, 14, 1622–1625 CrossRef CAS.
- H. Wang, G. Zhou and X. Chen, Sens. Actuators, B, 2013, 176, 698–703 CrossRef CAS.
- Y. Guo, F. Huo, C. Yin, J. Kang and J. Li, RSC Adv., 2015, 5, 10845–10848 RSC.
- X. Peng, J. Du, J. Fan, J. Wang, Y. Wu, J. Zhao, S. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500–1501 CrossRef CAS PubMed.
- D. Karak, S. Lohar, A. Banerjee, A. Sahana, I. Hauli, S. K. Mukhopadhyay, J. S. Matalobos and D. Das, RSC Adv., 2012, 2, 12447–12454 RSC.
- R. S. George, R. Joseph and K. George, Int. J. Polym. Mater., 1993, 23, 17–26 CrossRef CAS.
- S. Sen, T. Mukherjee, B. Chattopadhyay, A. Moirangthem, A. Basu, J. Marek and P. Chattopadhyay, Analyst, 2012, 137, 3975–3981 RSC.
- Z. C. Liao, Z. Y. Yang, Y. Li, B. D. Wang and Q. X. Zhou, Dyes Pigm., 2013, 97, 124–128 CrossRef CAS.
- H. Görner, S. Khanra, T. Weyhermüller and P. Chaudhuri, J. Phys. Chem. A, 2006, 110, 2587–2594 CrossRef PubMed.
- R. Peng, L. Lin, X. Wu, X. Liu and X. Feng, J. Org. Chem., 2013, 78, 11602–11605 CrossRef CAS PubMed.
- S. A. Lee, J. J. Lee, J. W. Shin, K. S. Min and C. Kim, Dyes Pigm., 2015, 116, 131–138 CrossRef CAS.
- W. Tang, Y. Xiang and A. Tong, J. Org. Chem., 2009, 74, 2163–2166 CrossRef CAS PubMed.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 68, 1414–1418 CrossRef CAS PubMed.
- N. E. Alstad, B. M. Kjelsberg, L. A. Vøllestad, E. Lydersen and A. B. Poléo, Environ. Pollut., 2005, 133, 333–342 CrossRef CAS PubMed.
- S. C. Burdette, G. K. Walkup, B. Spingler, R. Y. Tsien and S. J. Lippard, J. Am. Chem. Soc., 2001, 123, 7831–7841 CrossRef CAS PubMed.
- H. A. Benesi and J. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figures and tables. See DOI: 10.1039/c6ra02030e |
| This journal is © The Royal Society of Chemistry 2016 |