Effects of zinc and manganese ions in aqueous electrolytes on structure and electrochemical performance of Na0.44MnO2 cathode material†
Shouli Baia,
Jingli Songab,
Yuehua Wen*b,
Jie Chengb,
Gaoping Caob,
Yusheng Yangab and
Dianqing Li*a
aState Key Laboratory of Chemical Resource Engineering, Beijing Key Laboratory of Environmentally Harmful Chemicals Analysis, Beijing University of Chemical Technology, Beijing, China 100029. E-mail: lidq@mail.buct.edu.cn
bResearch Institute of Chemical Defence, Beijing, China 100191
First published on 13th April 2016
Abstract
The sodium manganese oxide, Na0.44MnO2, was synthesized by a solid-state reaction routine combined with a sol–gel process using Mn(CH3CO2)2·4H2O as the manganese source. Results show that the capacity and cycling stability of Na0.44MnO2 cathodes are enhanced significantly by using a hybrid aqueous electrolyte (Na2SO4, ZnSO4 and MnSO4). The energy storage mechanism of as-prepared Na0.44MnO2 in the hybrid aqueous electrolyte is associated with the insertion/extraction of zinc and sodium multi-ions with the help of synergistic effects between zinc and manganese ions and the quasi-reversible deposition–dissolution process of Mn2+ ions. The Na0.44MnO2 electrode displays both excellent storage properties with zinc, sodium and manganese ions (∼340 mA h g−1 at 100 mA g−1 after 150 cycles) and reversibility (∼100% coulombic efficiency during cycling). The excellent reversibility and good cycling properties indicate that the Na0.44MnO2 can be a promising material for energy storage devices by using a hybrid aqueous electrolyte.
1. Introduction
With the fast development of renewable energy sources such as solar energy and wind energy, safe energy storage battery systems with long-life, low-cost and eco-friendly options are urgently needed to fully utilize these energies, including their connection with smart grids. However, existing battery technologies cannot meet all the above needs and therefore the exploration of new energy storage and conversion systems is of great importance.In 2009, Kang et al.1 proposed a zinc ion battery (ZIB), which is composed of an α-MnO2 cathode, a zinc anode, and a mild ZnSO4 or Zn(NO3)2 aqueous electrolyte. The open circuit voltage (OCV) of the ZIB is approximately 1.5 V. At a current density of 0.2 A g−1, the MnO2 cathode of the ZIB delivers a capacity of 210 mA h g−1. In order to further improve the performance of MnO2 and increase the utilization ratio of MnO2, a α-MnO2/CNT nanocomposite was synthesized by Xu et al.2 In a mild aqueous electrolyte (ZnSO4 and MnSO4) the specific capacity of α-MnO2/CNT initially increased and then remained steady after a certain period of time with increase of cycling number. The composites display both excellent storage properties with zinc ions (∼665 mA h g−1 at 0.1 A g−1) and reversibility at various current rates. The specific capacity of the MnO2/CNT electrode gradually increases to be higher than the theoretical specific capacity of MnO2 converted from Mn(IV) to Mn(III). The author suggested that it might be attributed to a change in morphologies and structure of MnO2 and an improvement in the conductivity of MnO2. However, the weight ratio of carbon nanotube (CNT) to MnO2 is up to 20% in the MnO2/CNT composite. To prepare the cathode, carbon black (the conductor) was added at a weight ratio of 20%. In addition, the MnO2/CNT electrode was prepared by coating mixed slurry on steel foils with rather low mass of MnO2 in a piece of cathode. So the very high capacity is achieved only under special conditions. It was found by us that the above phenomenon can also be observed by using other cathodes such as LiMn2O4, LiFePO4 and even porous carbon electrodes etc. Therefore, the positive electrode materials are not limited to MnO2. The high specific capacity and excellent stability should mainly result from the effects of zinc and manganese ions in aqueous electrolytes.
With sodium’s high abundance and low cost, and suitable redox potential, rechargeable electrochemical cells based on sodium hold much promise for energy storage applications. Electrode materials with the orthorhombic Na0.44MnO2 structure show excellent reversibility towards alkali metal insertion processes.3–6 The studies of Na0.44MnO2 show that the specific capacity of Na0.44MnO2 in aqueous electrolyte is rather lower than that in organic electrolyte.4,7–10 Optimized Na0.44MnO2 was characterized by a pseudo-capacitive component, having a specific capacity of 30–45 mA h g−1 through a voltage range of 0.5 V in the aqueous electrolyte (1 M Na2SO4).4,8,9
Here we synthesized Na0.44MnO2 by a solid-state reaction routine combined with a sol–gel process using Mn(CH3CO2)2·4H2O as the manganese source. It was found that zinc and manganese ions in aqueous electrolytes have tremendous influence on the structure and electrochemical performance of the cathode materials. Employing a mild electrolyte containing sodium, zinc and manganese ions, the Na0.44MnO2 electrode showed both excellent cycle stability and high capacity.
2. Experimental
2.1 Preparation of Na0.44MnO2
Na0.44MnO2 was prepared by mixing Mn(CH3COO)2·4H2O (AR, 99%) and CH3COONa (AR, 99%) in de-ionized water at the ratio of Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 0.55. After rotary evaporation, the resulting powder was heated in air at 500 °C for 2 h at a heating rate of 5 °C min−1, followed by being fired at 800 °C for 16 h at a heating ramp rate of 2 °C min−1.
Mn = 0.55. After rotary evaporation, the resulting powder was heated in air at 500 °C for 2 h at a heating rate of 5 °C min−1, followed by being fired at 800 °C for 16 h at a heating ramp rate of 2 °C min−1.
2.2 Material characterizations
The crystal structure of Na0.44MnO2 powders was examined by a X-ray diffraction with a Shimadzu XRD-600 diffractometer, which was operated at 45 kV and 40 mA using Ni-filtered Cu Kα radiation (λ = 0.15406 nm), with a scanning speed of 10° min−1 for 2θ in a range from 10° to 60°. Surface elemental analysis of the samples was carried out using a S4800 scanning electron microscope with an electron dispersion spectroscope (SEM-EDS) attachment. Moreover, quantitative analysis of the Zn and Mn content in the electrolytic solution was achieved by inductively coupled plasma (NexION 300X).2.3 Electrochemical measurements
The as-prepared Na0.44MnO2 was mixed with acetylene black and poly(tetrafluoroethylene) (PTFE) in a weight ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with the help of ethanol. After drying, the mixture was pressed into a film, and then the film was cut into disks of about 1 × 1 cm2. These disks were pressed onto a stainless steel grid at a pressure of 30 MPa and then dried at 80 °C for one night and used as working electrodes.
5 with the help of ethanol. After drying, the mixture was pressed into a film, and then the film was cut into disks of about 1 × 1 cm2. These disks were pressed onto a stainless steel grid at a pressure of 30 MPa and then dried at 80 °C for one night and used as working electrodes.
Cyclic voltammetry (CV) measurements were performed on a Solartron model 1287 potentiostat/galvanostat system under ambient conditions. It is carried out by using a three-electrode cell, in which the Na0.44MnO2 electrode is used as the working electrode, zinc sheet as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. The electrolytes were 1 M Na2SO4, 1 M Na2SO4 + 0.05 M MnSO4, 1 M Na2SO4 + 0.5 M ZnSO4 and 1 M Na2SO4 + 0.5 M ZnSO4 + 0.05 M MnSO4 solutions, respectively.
Galvanostatic charge–discharge curves were recorded on a LAND CT2001A test system within 1.0–1.9 V using a two-electrode beaker-type cell, which was assembled with a Na0.44MnO2 cathode and a Zn anode. The active mass of the Na0.44MnO2 in a piece of cathode is about 5 mg while that of the zinc anode is excessive.
3. Results and discussion
3.1 Physicochemical characterizations
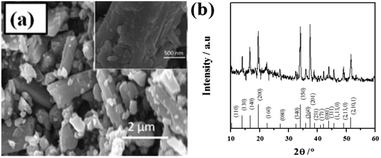
The SEM image of Na0.44MnO2 is shown in see Fig. 1a. The main constituent shape for the sample was rod-like crystals of approximately 2 μm long, in which there are certain amounts of small particles distributed. Fig. 1b shows the XRD pattern of as-prepared Na0.44MnO2. The XRD pattern well agrees with that of Na0.44MnO2 (JCPDS no. 27-0750). There were no discernible structures that corresponded to discrete impurities, suggesting the successful formation of Na0.44MnO2 with an orthorhombic lattice structure.3,8,113.2 Electrochemical properties
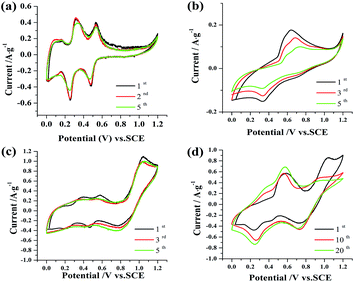
Fig. 2a shows the cyclic voltammograms of Na0.44MnO2 in 1 M Na2SO4 solution. The data shows two main redox couples at approximately 0.33 V/0.25 V and 0.53 V/0.47 V, respectively. These data are essentially identical to those reported by Whitacre8 for Na0.44MnO2 in an aqueous electrolyte (1 M Na2SO4), which corresponds to the insertion/de-insertion of sodium into lattice sites in the orthorhombic crystal structure. Moreover, the CV curve during the first cycle almost overlaps during the third cycle. But, the peak current in the fifth scan decreases slightly. After the addition of ZnSO4 into 1 M Na2SO4 solution, the cyclic voltammogram of Na0.44MnO2 changes dramatically as shown in Fig. 2b. The redox couples corresponding to the insertion/de-insertion of sodium cannot be observed. The oxidation/reduction peaks situated at 0.59 V and 0.25 V (vs. SCE) may be caused by the intercalation and de-intercalation of zinc ions.2,12,13 The peak current response of the electrode is not only lowered compared to that in 1 M Na2SO4 solution but also the peak current continuously decreases with increasing scans. It implies the insertion/de-insertion of zinc ions is more difficult than that of sodium ions. If MnSO4 is added to a 1 M Na2SO4 solution, two redox couples corresponding to the insertion/de-insertion of sodium can still be discernible as shown in Fig. 2c. These two redox couples tend to be ambiguous in subsequent scans. What’s more important is that a new redox couple situated at 1.0 V and 0.8 V (vs. SCE) appears. It can also be seen that the ratios of cathodic peak current to anodic peak current (Ipc/Ipa) is far from the value of unity and a broad cathodic peak is exhibited, indicative of a pseudo-reversible electrode process. At the same time, it may be influenced by the oxygen evolution side reaction. On the other hand, compared with the anodic process, the cathodic process kinetics are slower. Wei et al. suggested13 that Mn(II) ions might be oxidized to be MnOOH during charging, and then MnOOH turned to be MnO2 further by taking the following reaction: 2MnOOH + 2H+ ↔ MnO2 + Mn2+ + 2H2O. Thus, the redox process of Mn(II) ions is just reversible to a certain degree. With the scans increasing, the anodic peak current is reduced slightly while the cathodic peak current rises progressively. A greater change in the cyclic voltammogram of Na0.44MnO2 is presented when the mixed aqueous electrolyte of 1 M Na2SO4 + 0.5 M ZnSO4 + 0.05 M MnSO4 is employed, as shown in Fig. 2d. As can be seen, there are two redox couples, which are situated at 0.59/0.27 V and 1.0/0.78 V (vs. SCE), respectively. Compared with Fig. 2b, it is known that the redox peak at 0.59/0.27 V (vs. SCE) is attributable to zinc intercalation and deintercalation in the orthorhombic host structure in the aqueous electrolyte, which is similar to the behaviour found with MnO2 electrodes.14–16 Similarly, compared with Fig. 2c, the redox peak at 1.0/0.78 V (vs. SCE) may be ascribed to the oxidation/reduction of Mn2+ ions. However, in subsequent scans, the oxidation peak is lowered continuously and tends to be wider. In contrast, the reduction peak retains a well-defined shape and the current response rises to some extent. It suggests that the reduction peak at 0.78 V (vs. SCE) is also associated with zinc intercalation. Comparatively, the redox peak current at 0.59/0.27 V (vs. SCE) is enhanced considerably with the scans increasing, which may be caused by Mn(II) ions in the electrolytes.To examine the redox peak at 1.0/0.78 V vs. SCE, a graphite electrode with an area of 1 cm2 was employed to measure the cyclic voltammogram in 1 M Na2SO4 + 0.05 M MnSO4, the result is shown in Fig. 3. It can be seen that the redox peak at 1.0/0.78 V vs. SCE is similar to that at Fig. 2c. A pseudo-capacitance is exhibited in the potential range of 0–0.7 V vs. SCE. Since the graphite electrode is inert, it is demonstrated that the redox peak at 1.0/0.78 V should be ascribed to the oxidation and reduction of Mn2+ ions.
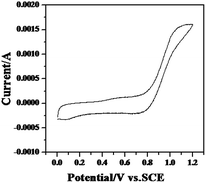 | ||
| Fig. 3 Cyclic voltammogram of a graphite electrode in the solution of 1 M Na2SO4 + 0.05 M MnSO4, scan rate: 1 mV s−1. | ||
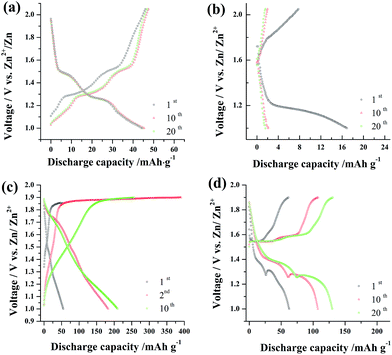
Fig. 4a shows galvanostatic charge–discharge cycling curves collected from a three-electrode cell with activated carbon and zinc electrodes as counter and reference electrodes. The relative potential and shape of the two largest potential plateaus observed for the Na0.44MnO2 positive electrode in 1 M Na2SO4 are consistent with the CV data shown in Fig. 2a and also agree with those published in the literature for this material in various other aqueous electrolytes.4,8 Fig. 4b shows the discharge/charge profiles of the Na0.44MnO2 electrode in 1 M Na2SO4 + 0.5 M ZnSO4 at the 1st, 10th and 20th cycles. It can be seen that the discharge process during the first cycle is rather different from the ones observed in subsequent cycles. The discharge capacity in the initial cycle is much larger than those in the subsequent cycles. This may be associated with the original Na0.44MnO2 electrode at a high valence, which is confirmed by the open-circuit voltage of up to 1.55 V before charge–discharge cycles. After the first cycle, the Na0.44MnO2 electrode suffers from rapid capacity fading and the charge–discharge curves exhibit a capacitive behaviour. It implies that the crystal structure of Na0.44MnO2 may be changed and a high resistance is expected for Na+/Zn2+ ions insertion and extraction. To illustrate this phenomenon, the XRD pattern of the Na0.44MnO2 electrode after 20 cycles in 1 M Na2SO4 + 0.5 M ZnSO4 mixed aqueous electrolytes is shown in the ESI (Fig. S1†). As can be seen, some characteristic diffraction peaks of Na0.44MnO2 are evidently weakened, indicative of a great change in the crystal structure of Na0.44MnO2. The radius of a Zn2+ ion is only 0.6 Å, which is almost the same as that of a Li+ ion (0.59 Å). While, the Na+ ionic radius is as large as 0.97 Å. Thus, it may be inferred that in the solution containing Zn2+ ions, some Zn2+ ions are inserted into Na0.44MnO2 by ion-exchange-type reaction due to the relatively low resistance of zinc insertion. As a result, the crystal structure of Na0.44MnO2 partly collapses due to the effect of Zn2+ ions. This results in the rather low capacity and a dramatic drop in the capacity of the Na0.44MnO2 electrode in 1 M Na2SO4 + 0.5 M ZnSO4 solution. When 0.05 M MnSO4 is added into 1 M Na2SO4 solution, the capacity is enhanced significantly, as shown in Fig. 4c. A pseudo-capacitive behaviour is displayed. When the charge cut-off voltage is 1.85 V in the initial cycle, the Na0.44MnO2 electrode presents a small voltage plateau of about 1.85 V at the charge curve end. The discharge capacity and the coulombic efficiency are 54 mA h g−1 and nearly 100%, respectively. In subsequent cycles, the charge cut-off voltage is increased to 1.9 V. A large voltage plateau of charge between 1.85 V and 1.9 V is exhibited. The coulombic efficiency is reduced to less than 90%. The discharge capacity is enhanced considerably owing to the overcharge process. With the cycling number increasing, the discharge capacity increases slowly and the coulombic efficiency rises continuously to nearly 90%. The voltage plateau at charge curve end tends to be smaller. This agrees with the pseudo-reversible electrode process of Mn2+ ions as shown in Fig. 2c. Employing the mixed aqueous solution of 1 M Na2SO4 + 0.5 M ZnSO4 + 0.05 M MnSO4, a great change takes place in the charge–discharge profiles for Na0.44MnO2, as shown in Fig. 4d. Though the charge cut-off voltage is still 1.9 V, two charge plateaus at around 1.55/1.59 V (vs. Zn/Zn2+) and two discharge plateaus at 1.35/1.25 V (vs. Zn/Zn2+) tend to be more significant with the increase of cycling number in the presence of zinc ions. Thus, the capacity of Na0.44MnO2 cathode increases markedly with the cycling number. Synchronously, the coulombic efficiency maintains nearly 100% during cycling. The capacity of the Na0.44MnO2 cathode rises up to 130 mA h g−1 at just the 20th cycle. This may be ascribed to one kind of synergistic effect between zinc and manganese ions. In addition, the effect of quasi-reversible deposition/dissolution process of Mn2+ ions can also not be excluded. These observations are consistent with the cyclic voltammogram results shown in Fig. 2d.
CV curves of Na0.44MnO2 with different scan rates in 1 M Na2SO4 + 0.5 M ZnSO4 + 0.05 M MnSO4 mixed aqueous electrolyte are presented in Fig. 5a. The CV of as prepared Na0.44MnO2 cathodes at the scan rate of 0.1 mV s−1 displays two separated pairs of redox peaks, which are situated at 1.7/1.3 V and 1.6 V/1.1 V (vs. Zn/Zn2+). According to the above CVs, they correspond to the two stages of intercalation and de-intercalation of Zn2+ ions in aqueous electrolytes. With the increase of the scan rate, the peak separation increases and two separated redox peaks are combined progressively due to over potentials. Moreover, the peaks cannot retain the defined shape when the scan rate increases to 30 mV s−1 due to the relatively slow kinetic properties of Zn2+ ions in the electrode. The corresponding rate performance of Na0.44MnO2 in this hybrid electrolyte is shown in Fig. 5b. The Na0.44MnO2 shows an initial discharge capacity of 250 mA h g−1 at the current density of 100 mA g−1, only 83.3 mA h g−1 at the current density of 600 mA g−1. The capacities at various rates present relatively rapid graded changes. But, the recovered capacity is a little higher than the initial capacity. It suggests that the Na0.44MnO2 cathode could be capable of reversible charge and discharge, but its rate capability needs to be improved further. The cycling performance of the Na0.44MnO2 cathode at the current density of 100 mA g−1 is shown in Fig. 5c. It is found that the capacity of Na0.44MnO2 initially increased dramatically and then remained steady after a period of time with increase of cycling number. The capacity finally remained steady at about 340 mA h g−1 after 150 cycles, which is higher than the theoretical specific capacity (276 mA h g−1) of Na0.44MnO2 converted from Mn(IV) to Mn(III). The high capacity and excellent cycling performance of the Na0.44MnO2 electrode might result from a synergistic effect between zinc and manganese ions as shown in the CV curves of Na0.44MnO2 (see Fig. 2). In addition, as seen in Fig. 4c, when the charge voltage is above 1.85 V (vs. Zn/Zn2+), the pseudo-reversible redox reaction of Mn2+ ions may occur. On the other hand, it is seen from the SEM images of the Na0.44MnO2 electrode before and after cyclic tests that Na0.44MnO2 obviously changes from a compact rod-like to sponge-like structure with increase of cycling number (see Fig. 5d). The sponge-like Na0.44MnO2 with porous structure after cycling may result in a large increase of active surface area of the electrode, which can absorb and store more zinc and sodium ions. As a result, the utilization of the Na0.44MnO2 electrode and capacity are improved to a large extent.
To investigate the electrochemical shuttling mechanism of Mn2+ and Zn2+ ions, which may provide the extra capacity further, the concentration of ion (Mn2+, Zn2+) in the electrolyte as a function of cycling number was determined. The results are listed in Table 1. As can be seen, after 20 charge–discharge cycles, the concentration of Mn2+ ions is almost unchanged and the concentration of Zn2+ ions is reduced slightly to 0.49 M. In contrast, the concentration of Mn2+ ions decreases from 0.052 M to 0.031 M, while the concentration of Zn2+ ions is reduced significantly to be 0.28 M after prolonged cycling of 170. From Fig. 3, it is confirmed that the redox peak at 1.0/0.8 V vs. SCE is ascribed to the oxidation and reduction of Mn2+ ions. The electro-oxidation of Mn(II) ions may be written as: Mn2+ + 2H2O − e− ↔ MnOOH + 3H+. And then MnOOH is converted to MnO2 by taking a disproportionation reaction further. Thus, the redox process of Mn(II) ions is reversible to a certain degree. As a result, some deposited MnO2 may prevent the Mn dissolution of Na0.44MnO2 during charge and discharge. At the same time, the deposited MnO2 may provide a certain amount of extra capacity after prolonged cycling. That is why the finally maintained steady capacity (about 340 mA h g−1) is higher than the theoretical specific capacity (276 mA h g−1) of Na0.44MnO2 converted from Mn(IV) to Mn(III) after prolonged cycling. However, when the capacity is maintained steady, the Mn(II) ions in solution should be at a state of dynamic equilibrium. A large decrease in the concentration of Zn2+ ions is directly related to the zinc anode. Since the zinc anode is used, the energy storage of the anode is realized by a deposition and dissolution process. Thus, after prolonged cycling, some zinc powder might fall off from the zinc anode. At the same time, a little basic zinc sulfate was also formed. This has been confirmed by the literature.17
| Cycle number | Mn2+/mol l−1 | Zn2+/mol l−1 |
|---|---|---|
| 0 | 0.05185 | 0.51874 |
| 20 | 0.05 | 0.48769 |
| 170 | 0.03121 | 0.2769 |
The XRD patterns of Na0.44MnO2 electrodes in the extraction and insertion states are shown in Fig. 6. It is found that the positions and strengths of most peaks for Na0.44MnO2 have evidently not been changed. In the insertion state, the high and sharp peak at 12° and the small peak at 25° may be attributed to the Na0.91MnO2. The formation of ZnMn2O4 and Na0.91MnO2 confirms the insertion of Zn2+ and Na+ multiple ions into Na0.44Mn2O4. Compared with the original Na0.44MnO2, the other new diffraction peaks might be the formation of a mixed oxide including Na, Zn and Mn. In the extraction state, most diffraction peaks of Na0.44MnO2 are remained, only being shifted a bit to the right. Two small weak peaks at about 12° and 33° belonging to Na0.91MnO2 and ZnMn2O4 can still be observed. It is demonstrated that the insertion/extraction mechanism of zinc and sodium multiple ions into/from the crystalline Na0.44MnO2 during charge and discharge in the hybrid aqueous electrolyte (Na2SO4, ZnSO4 and MnSO4).
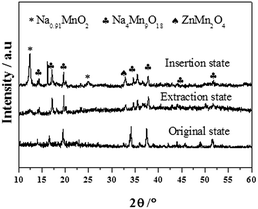 | ||
| Fig. 6 XRD patterns of the Na0.44MnO2 electrode before and after 50 cycles at the current density of 100 mA g−1 in 1 M Na2SO4 + 0.5 M ZnSO4 + 0.05 M MnSO4 mixed aqueous electrolytes. | ||
The pristine and cycled cathodes at charge and discharge states are measured by EDS elemental analysis, further. Fig. 7 shows the EDS elemental distribution of Zn, Mn, Na and C species on the pristine and cycled cathode surface at charge and discharge states. The corresponding atomic percents are listed in Table 2. It can be seen that the appearance of C elemental peak is attributed to the addition of carbon black in the process of preparing the Na0.44MnO2 electrode. At the discharge state of cycled cathode, the relative atomic percents of Zn and Na increase greatly compared to that at the charge state, especially for the Zn element. Correspondingly, the relative atomic percentage of Mn at the discharge state of cycled cathode is sure to decrease considerably compared to that at the charge state. Thus, it is demonstrated that the insertion/extraction of zinc and sodium multi-ions from Na0.44MnO2 surely takes place, and most of the capacity is achieved by the insertion/extraction of zinc ions.
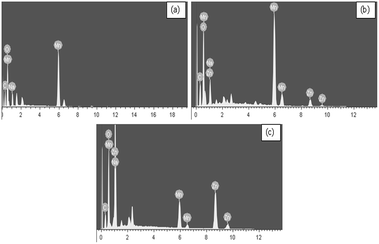 | ||
| Fig. 7 EDS spectra of Zn, Mn, Na and C species on the pristine (a) and cycled cathode surface at charge state (b) and discharge state (c) of the 20th cycle. | ||
| States | Mn/% | Zn/% | Na/% | C/% |
|---|---|---|---|---|
| Original state | 16.58 | 0 | 6.32 | 39.62 |
| Discharge state of the 20th cycle | 4.23 | 14.14 | 6.83 | 45.23 |
| Charge state of the 20th cycle | 15.59 | 2.86 | 3.15 | 37.01 |
4. Conclusions
The sodium manganese oxide, Na0.44MnO2, was synthesized by a solid-state reaction routine combined with a sol–gel process using Mn(CH3CO2)2·4H2O as the manganese source. By using a hybrid aqueous electrolyte (Na2SO4, ZnSO4 and MnSO4), the capacity and cycling stability of Na0.44MnO2 cathodes are enhanced significantly. It is found that the capacity of Na0.44MnO2 initially increased and then remained steady after a certain period of time with increase of cycling number. It rises up to 340 mA h g−1 after 150 cycles at the current density of 100 mA g−1 within the range from 1 to 1.9 V (vs. Zn/Zn2+) of the potential window. The morphology of Na0.44MnO2 changes from a compact rod-like to loose sponge-like porous structure during charge and discharge. The electrochemical measurements shows that the as-prepared Na0.44MnO2 cathode in the hybrid aqueous electrolyte reveals the insertion/extraction mechanism of zinc and sodium ions into/from the crystalline Na0.44MnO2 with the help of synergistic effects between zinc and manganese ions during the energy storage process. In addition, the effect of a quasi-reversible deposition/dissolution process of Mn2+ ions is also included. The excellent reversibility and good cycling properties indicate that the Na0.44MnO2 can be a promising material for energy storage devices in the hybrid aqueous electrolyte.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 51372013), the Fundamental Research Funds for the Central Universities (YS1406), the Beijing Engineering Center for Hierarchical Catalysts and Beijing Key Laboratory of Advanced Chemical Energy Storage Technology and Material (Z121109002812019).Notes and references
- C. Xu, H. Du, B. Li, F. Kang and Y. Zeng, Electrochem. Solid-State Lett., 2009, 12, A61–A65 CrossRef CAS.
- D. Xu, B. Li, C. Wei, Y.-B. He, H. Du, X. Chu, X. Qin, Q.-H. Yang and F. Kang, Electrochim. Acta, 2014, 133, 254–261 CrossRef CAS.
- J.-H. Lee, R. Black, G. Popov, E. Pomerantseva, F. Nan, G. A. Botton and L. F. Nazar, Energy Environ. Sci., 2012, 5, 9558 CAS.
- D. J. Kim, R. Ponraj, A. G. Kannan, H.-W. Lee, R. Fathi, R. Ruffo, C. M. Mari and D. K. Kim, J. Power Sources, 2013, 244, 758–763 CrossRef CAS.
- L. Zhao, J. Ni, H. Wang and L. Gao, RSC Adv., 2013, 3, 6650 RSC.
- R. Ruffo, R. Fathi, D. J. Kim, Y. H. Jung, C. M. Mari and D. K. Kim, Electrochim. Acta, 2013, 108, 575–582 CrossRef CAS.
- H.-X. Yang and J.-F. Qian, J. Inorg. Mater., 2013, 28, 1165–1171 CrossRef CAS.
- J. F. Whitacre, A. Tevar and S. Sharma, Electrochem. Commun., 2010, 12, 463–466 CrossRef CAS.
- A. D. Tevar and J. F. Whitacre, J. Electrochem. Soc., 2010, 157, A870 CrossRef CAS.
- M. M. Doeff, T. J. Richardson and K.-T. Hwang, J. Power Sources, 2004, 135, 240–248 CrossRef CAS.
- E. Hosono, T. Saito, J. Hoshino, M. Okubo, Y. Saito, D. Nishio-Hamane, T. Kudo and H. Zhou, J. Power Sources, 2012, 217, 43–46 CrossRef CAS.
- C. Xu, B. Li, H. Du and F. Kang, Angew. Chem., 2012, 51, 933–935 CrossRef CAS PubMed.
- C. Wei, C. Xu, B. Li, H. Du and F. Kang, J. Phys. Chem. Solids, 2012, 73, 1487–1491 CrossRef CAS.
- M. H. Alfaruqi, J. Gim, S. Kim, J. Song, J. Jo, S. Kim, V. Mathew and J. Kim, J. Power Sources, 2015, 288, 320–327 CrossRef CAS.
- C. Yuan, Y. Zhang, Y. Pan, X. Liu, G. Wang and D. Cao, Electrochim. Acta, 2014, 116, 404–412 CrossRef CAS.
- C. Xu, B. Li, H. Du and F. Kang, Angew. Chem., Int. Ed., 2012, 51, 933–935 CrossRef CAS PubMed.
- H. Li, Master Thesis, Tsinghua University, 2012.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra01768a |
| This journal is © The Royal Society of Chemistry 2016 |