Cd-doping a facile approach for better thermoelectric transport properties of BiCuSeO oxyselenides
Abstract
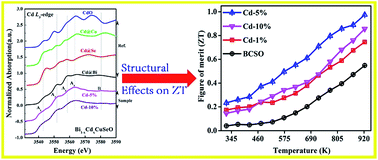
BiCuSeO-based thermoelectric materials have spurred tremendous interest among the thermoelectric community due to their ultra-low thermal conductivity and relatively large Seebeck coefficient (S). In this work, we have reported the effect of Cd-doping at the Bi site, instead of the previously studied Cu site, on the thermoelectric performance of BiCuSeO by modifying the insulating layer. While maintaining good phase purity, Cd was successfully doped at the Bi site as confirmed by X-ray absorption fine structure spectroscopy. The Cd-doping substantially improves the electrical conductivity by a factor of 20 through bond anharmonicity at room temperature while increasing the Cd concentration over 5%. Further, the incorporation of the lighter atom at the Bi site creates phonon scattering centers and results in weak bonding between the layers, resulting in a remarkable perturbation of the local geometric and electronic structure. BiCuSeO with 5% Cd-doping maintains a large S and a high electrical conductivity up to 923 K and exhibits the highest power factor values (600 μW m−1 K−2 at 323 K and 447 μW m−1 K−2 at 923 K) and the largest ZT (0.98 at 923 K). Cd-doping at the Bi site in p-type thermoelectric BiCuSeO was shown to be a very good technique for improving the thermoelectric performance and could be extended to other thermoelectric materials to enhance the efficiency of thermoelectric devices for energy-harvesting.

 Please wait while we load your content...
Please wait while we load your content...