A single chemosensor for bimetal Cu(II) and Zn(II) in aqueous medium†
Zhuang Liaoa,
Dan Wanga,
Jian-Quan Zhengb,
Hong-Wei Tana,
Xiang-Jun Zheng*a and
Lin-Pei Jina
aBeijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing, 100875, Peoples' Republic of China.. E-mail: xjzheng@bnu.edu.cn
bBeijing Key Laboratory for Bioactive Substances and Functional Foods, Beijing Union University, Beijing, 100191, People's Republic of China
First published on 30th March 2016
Abstract
A new quinazoline derivative 6-(4-diethylamino)phenol-2-yl-(5,6-dihydrobenzimidazo[1,2-c])-quinazoline (HL) and the two metal Schiff-base complexes [CuL1Cl]·CH3OH (1) and [ZnL1(Ac)]·0.5CH3CH(OH)CH3 (2) (HL1 = 4-{[2-(1H-benzoimidazol-2-yl)-phenyl imino]-methyl}-benzene-1-diethylamino-3-ol) were synthesized and characterized by single-crystal X-ray diffraction. HL is highly selective and sensitive to Cu(II) and Zn(II) ions in aqueous medium with a detection limit of 2.13 × 10−6 and 7.19 × 10−7 M for Cu2+ and Zn2+, respectively. HL can act as a “turn-off” sensor for Cu(II) with the excitation wavelength of 356 nm and a “turn-on” sensor for Zn(II) when excited at 416 nm. The Job plots, fluorescence and UV-vis titrations, 1H NMR as well as ESI data show that the binding stoichiometry of Cu(II)/Zn(II) with HL is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The binding sites of HL with Cu(II)/Zn(II) are two nitrogen atoms and one oxygen atom, forming Cu(II)–L1 and Zn(II)–L1 complexes. This agrees with the crystal structures of 1 and 2.
1. The binding sites of HL with Cu(II)/Zn(II) are two nitrogen atoms and one oxygen atom, forming Cu(II)–L1 and Zn(II)–L1 complexes. This agrees with the crystal structures of 1 and 2.
Introduction
Acting as the most active and innovative force in the ion detection field, fluorescent sensors have many advantages, such as high selectivity, high sensitivity and simple synthetic methods.1 Copper is the third most abundant transition metal in the human body,2 but excessive copper can lead to serious neurological diseases.3 What's more, if copper is discharged into the environment, it will cause pollution. Zinc is the second most abundant transition metal in the body4 and plays an important role in many living systems. Researches show that zinc is essential for enzyme functions, can stabilize the cell membrane, resist free radicals5 and also can recognize DNA or RNA.6 The disorder of zinc can cause some health problems like Alzheimer's disease, prostate cancer and diabetes.7 So researches on chemosensors for Cu(II) and Zn(II) are much important in both life and environmental sciences.Currently, a large number of fluorescent sensors for Cu(II) or Zn(II) have been designed and synthesized.8 The observations show that chemosensors for detection of metal ions are dependent on nature of metal ions, basicity of the donor atoms, solvents and pH. Recently, a new chemosensor strategy of “single chemosensor for multiple metals” has emerged. This can be realized by the above considerations.9 It is obvious that a single chemosensor probes multiple metal ions, it will have better application foreground. In comparison, single chemosensors for the detection of Cu(II) and Zn(II) at trace quantity levels by fluorescence quenching and enhancement have been rarely reported.10 Thus, it is still a challenge to develop single chemosensor for Cu(II) and Zn(II) ions.
The quinazoline derivative is an ideal platform for development of chemosensors for transition metal ions such as Zn2+, Cu2+, Hg2+, Pb2+, Al3+, Cr3+ and Fe3+.11 In general, the quinazoline derivatives are coordinated to metal ions, metal Schiff-base complexes will be resulted.11,12 In most cases, the emission band positions will obviously change. As is known, the introduction of strong electron donating groups to the quinazoline derivatives can change their electron structures and thus their fluorescent properties. For example, the diethylamino group (–NEt2) which is a strong electron donating group can improve the optical properties of the metal complexes, such as Stokes shifts.13 To further explore the quinazoline derivative as a single chemosensor for multi-metal ions, we report a new quinazoline compound, 6-(4-diethyl amino)phenol-2-yl-(5,6-dihydrobenzimidazo-[1,2-c])-quinazoline (HL) synthesized by the condensation of 2-(2-amino-phenyl)benzimidazole with 4-(diethylamino)salicylaldehyde. HL is a chemosensor for detection of both Cu(II) and Zn(II) ions by the selective excitation. Furthermore, HL can be used to detect intracellular Cu(II) ion.
Results and discussion
Spectra and crystal structure of HL
The UV-vis spectral observations were conducted in DMSO/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). UV-vis spectrum of HL shows π → π* transition at 292 and 302 nm while n → π* transition locates at 354 nm, shown as in Fig. S1.† From the excitation and fluorescence spectra of HL (Fig. 1a), we can see that no matter the excited wavelength is 270 nm or 356 nm, an emission band with maximum intensity at 442 nm appears. The crystal structure of HL is shown in Fig. 1b. The results show that the molecule is not coplanar and there are intermolecular hydrogen bonds in HL (Fig. S2†). HL has two potential coordination sites, O1, N2 and N4 as shown in Fig. 1b.
1, v/v). UV-vis spectrum of HL shows π → π* transition at 292 and 302 nm while n → π* transition locates at 354 nm, shown as in Fig. S1.† From the excitation and fluorescence spectra of HL (Fig. 1a), we can see that no matter the excited wavelength is 270 nm or 356 nm, an emission band with maximum intensity at 442 nm appears. The crystal structure of HL is shown in Fig. 1b. The results show that the molecule is not coplanar and there are intermolecular hydrogen bonds in HL (Fig. S2†). HL has two potential coordination sites, O1, N2 and N4 as shown in Fig. 1b.
 | ||
| Fig. 1 (a) Excitation and emission spectra of HL (10 μM) in DMSO/H2O (1/1, v/v); (b) crystal structure of HL. | ||
Selectivity of HL to metal ions
A series of solutions were prepared with 10 μM HL and 1.0 equiv. metal ions including Al3+, Zn2+, Cd2+, Co2+, Ca2+, Cr3+, Mn2+, Ni2+, Fe2+, Cu2+, Pb2+, Na+, K+, Li+, Hg2+ and Mg2+ in DMSO/H2O (1/1, v/v). When excited at 356 nm, only Cu(II) resulted in fluorescence quenching of HL, the fluorescence intensity was reduced 20-fold at 425 nm (Fig. 2a). This means that HL can be a “turn-off” sensor for Cu(II). In the case of Zn(II) ion, the excitation and fluorescence spectra of HL + 1.0 equiv. Zn(II) were obtained (Fig. S3†), two bands at 425 nm and 481 nm could be observed when excited at 356 nm. While only one intensive emission band at 481 nm was observed when excited at 416 nm. So 416 nm was selected as excitation wavelength to observe the sensing properties of HL to metal ions. We got a gratifying result, only Zn(II) has a 17-fold fluorescence enhancement at 481 nm (Fig. 2b). This means that HL has selectivity to Cu(II) and Zn(II) by using the excitation wavelength 356 nm and 416 nm, respectively. | ||
| Fig. 2 Emission spectra of HL (10 μM) upon addition of 1 equiv. metal ions in DMSO/H2O (1/1, v/v): (a) λex = 356 nm; (b) λex = 416 nm. | ||
The competition experiments were carried out to test the selectivity of HL to Cu(II) and Zn(II) in the presence of other metal ions Al3+, Cd2+, Co2+, Ca2+, Cr3+, Mn2+, Ni2+, Fe2+, Pb2+, Na+, K+, Li+, Hg2+ and Mg2+ as shown in Fig. S4.† Fig. S4a† shows that the existence of other metal ions will not interfere the detection of Cu(II). From Fig. S4b,† we can see that other metal ions are not interferential to the detection of Zn(II) except Cu(II). As the literature reported, S2− could be used to shelter Cu(II) when Zn(II) was detected.14 Thus, HL is highly sensitive to Zn2+ ion.
Binding mode and species of HL with metal ions
Job plots of HL to Cu(II) and HL to Zn(II) were carried out by UV-vis spectra and fluorescence spectra in DMSO/H2O (v/v, 1/1), respectively. From Job plots we can see that the binding stoichiometry for HL to both of Cu(II) and Zn(II) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3). The Job plot of HL to Zn(II) can also be carried out by UV-vis spectra (Fig. S5†). 1
1 (Fig. 3). The Job plot of HL to Zn(II) can also be carried out by UV-vis spectra (Fig. S5†). 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for HL to Zn(II) was obtained.
1 binding stoichiometry for HL to Zn(II) was obtained.
The assignments of 1H NMR signals of HL and 1H NMR titration of HL with Zn(II) in DMSO-d6 were established, respectively (Fig. 4). From Fig. 4a, the hydrogen atoms of HL except two ethyl groups are all identified. When 0.5 equiv. Zn(II) was added to HL (Fig. 4b), signals at 9.66 ppm (H1) and 7.10 ppm (H5) of two active protons decreased. While two new signals at 5.87 ppm and 6.08 ppm for H2 and H3 of phenol group appeared, respectively (Fig. 4b). This shows that Zn(II) complex and HL coexisted. Comparing Fig. 4a with c, we can see that the signals of active protons of O–H (H1) at 9.66 ppm and N–H (H5) at 7.10 ppm disappeared after the addition of 1.0 equiv. Zn(II) to HL, indicating the O and N atoms from HL bind with Zn(II). Meanwhile, the proton H2 at 6.17 ppm shifted to high field (5.87 ppm) and H3 at 5.98 ppm shifted to low field (6.08 ppm), signals for phenyl ring protons shift to low field. These are attributed to the coordination of two N atoms and one O atom from HL with Zn(II). The signals of Fig. 4c and d are very similar, proving the binding stoichiometry of HL with Zn(II) to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 once again.
1 once again.
 | ||
| Fig. 4 1H NMR spectra in DMSO-d6: (a) HL; (b) HL + 0.5 equiv. Zn2+; (c) HL + 1.0 equiv. Zn2+; (d) HL + 1.5 equiv. Zn2+. | ||
Additionally, electrospray ionization mass spectra were carried out to study the species formed in the solution, the results are shown in Fig. S6.† After Cu(II) was added to HL, the intense peak at m/z = 446.8 (calcd 446.1) can be observed attributed to [CuL1]+. When Zn(II) was added to HL in DMSO/H2O (v/v, 1/1), intense peaks at m/z = 446.6 (calcd 447.1) and 488.2 (calcd 488.1) appeared, which are attributed to [ZnL1]+ and [ZnL1(CH3CN)]+, respectively. The results proved the binding stoichiometry for HL with both of Zn(II) and Cu(II) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
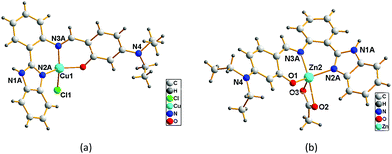
To further explore the species formed in the response system of HL with Cu(II) and Zn(II), we synthesized the Cu(II) and Zn(II) complexes with HL. The crystal structures show that either one-pot reaction or the direct reaction of Cu2+ with HL, the Schiff base complexes were resulted. In 1, Cu(II) ion is four-coordinated with two N atoms and one O atom from L1 and one chlorine atom, shown as in Fig. 5a. In 2, Zn(II) ion is five-coordinated with two N atoms and one O atom from L1, two oxygen atoms from Ac−, as shown in Fig. 5b. Actually, in 2 there are two very similar Zn2+ ions with slightly different bond lengths and angles (Table S3†). The crystal structures of 1 and 2 and the above 1H NMR observations prove that the binding mode of L1 with Zn(II) is the same both in solid and solution. In addition, we carried out the 1H NMR measurement for the synthesized Zn(II) complex and HL + 1.0 equiv. Zn(II) system (Fig. S7†). The two spectra showed basically the same, supporting that the coordinated species formed in the system of HL + 1.0 equiv. Zn(II) may be the same as that of the synthesized Zn(II) complex (2). This is also confirmed by the fact that UV-vis spectra of 2 and HL + 1.0 equiv. Zn(II) are the same, as shown in Fig. S8a.† Because Cu2+ is paramagnetic, high resolution 1H NMR of Cu2+ species could not be observed. However, from Fig. S8b† we can believe that the Cu(II) complex (1) was formed in the system of HL + 1.0 equiv. Cu(II). In a word, the above mentioned UV-vis and emission spectra, 1H NMR, EMS and single-crystal diffraction data confirm that complexes 1 and 2 were formed in the response system of HL with Zn(II) and Cu(II) in DMSO/H2O (1/1, v/v), respectively. Due to the formation of Schiff base complexes, the maximum absorption bands of Cu2+ and Zn2+ response systems are red-shifted to 420 nm (Fig. S8†), and the emission peak of Zn2+ response system is red-shifted to 481 nm. The introduction of diethylamino group (–NEt2) to the salicylaldehyde moiety makes the emission peak of Zn(II) complex red-shifted about 60 nm.12a While the fluorescence quenching of HL by Cu(II) is attributed to LMCT.12b
Sensitivity of HL to Cu(II) and Zn(II) ions
HL sensor's titration experiments were carried out (Fig. 6). In Fig. 6a, as the addition of Cu(II) to HL, a major absorption peak appeared at 418 nm. The absorbance increased gradually with the increasing of the Cu(II) concentration, and it tends to be maximum when molar ratio of HL to Cu(II) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 6b shows that the fluorescence intensity of HL with Zn(II) at 481 nm increases with the increasing of the Zn(II) concentration. The fluorescence intensity is apt to be the same when molar ratio of HL to Zn(II) is over 1
1. Fig. 6b shows that the fluorescence intensity of HL with Zn(II) at 481 nm increases with the increasing of the Zn(II) concentration. The fluorescence intensity is apt to be the same when molar ratio of HL to Zn(II) is over 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The binding constants of HL with Cu(II) and Zn(II) were calculated with the formula of Benesi–Hildebrand.15 For Cu(II), the binding constant (log
1. The binding constants of HL with Cu(II) and Zn(II) were calculated with the formula of Benesi–Hildebrand.15 For Cu(II), the binding constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) was 6.09 × 104 M−1 and the detection limit for Cu(II) was 2.13 × 10−6 M (Fig. S9†). For Zn(II), the binding constant (log
Ka) was 6.09 × 104 M−1 and the detection limit for Cu(II) was 2.13 × 10−6 M (Fig. S9†). For Zn(II), the binding constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka) was 1.12 × 106 M−1 and the detection limit was 7.19 × 10−7 M (Fig. S10†). The comparison of the detection limits of HL for Cu2+ and Zn2+ with other quinazoline derivatives in addition to the referred chemosensors shows that the detection limit of Cu2+ for this work is lower than 7.1 × 10−6 M,12b but higher than 1.82 × 10−8 M.12c The detection limit of Zn2+ is higher than 1.58 × 10−10 M.11a The reason for difference of the detection limits could be mainly attributed to the component and the structure of the raw aldehydes, and then the structure of the synthesized quinazoline derivatives.
Ka) was 1.12 × 106 M−1 and the detection limit was 7.19 × 10−7 M (Fig. S10†). The comparison of the detection limits of HL for Cu2+ and Zn2+ with other quinazoline derivatives in addition to the referred chemosensors shows that the detection limit of Cu2+ for this work is lower than 7.1 × 10−6 M,12b but higher than 1.82 × 10−8 M.12c The detection limit of Zn2+ is higher than 1.58 × 10−10 M.11a The reason for difference of the detection limits could be mainly attributed to the component and the structure of the raw aldehydes, and then the structure of the synthesized quinazoline derivatives.
The detection of intracellular Cu(II) ion
In order to certify whether HL can probe intracellular Cu2+ ion, we carried out the cell imaging experiments. Before imaging, the SH-SY5 cells were incubated with CuCl2 for 8 h and then added HL (20 μM) to it, incubated for 30 min. From Fig. 7, we can see that fluorescence of HL was observed; when cells exposed to HL with Cu(II), no fluorescence was observed. These experimental results show that HL has the ability to permeate cells and binds with Cu(II). So HL can detect intracellular Cu(II).Experimental section
Materials and instrumentation
All solvents and reagents are of analytical grade and were used as received. Metal salts were Mn(ClO4)2·6H2O, CrCl3·6H2O, Ni(ClO4)2·6H2O, Al(ClO4)3·9H2O, Fe(ClO4)3·9H2O, Co(ClO4)2·6H2O, Cd(ClO4)2·6H2O, Hg(ClO4)2·3H2O, Pb(ClO4)2·3H2O, Zn(Ac)2·2H2O, LiCl, NaCl, KNO3, CaCl2, MgCl2·6H2O and CuCl2·2H2O. The UV-vis absorption and fluorescence spectra were obtained from UV-2450 spectrophotometer and Cary Eclipse fluorescence spectrophotometer, respectively. C, H and N elemental analyses were measured with a Vario EL elemental analyzer. Fourier transform infrared (FT-IR) spectra were recorded on an Avatar 360 FT-IR spectrometer as KBr pellets. 1H NMR spectra were collected on a Bruker Avance III 400 MHz spectrometer. ESI mass spectra (ESI) were obtained on a Quattro micro API mass spectrometer.Preparation of HL
4-(Diethylamino)salicylaldehyde (0.0386 g, 0.2 mmol) and 2-(2-aminophenyl)benzimidazole (0.0418 g, 0.2 mmol) were mixed in 2 mL ethanol in a 25 mL Teflon-lined autoclave, heated at 80 °C for 1 day, and then cooled to room temperature. Light-claybank prismatic crystals of HL were obtained, the yield was 78.0%. Anal. calcd for C24H24N4O: C, 74.97; H, 6.29, N, 14.57. Found: C, 74.74; H, 6.31; N, 14.54. IR (KBr pellet, cm−1): 3414s, 1618vs, 1533m, 1500s, 1383s, 1252m, 1105m, 745s.Synthesis of [CuL1Cl]·CH3OH (1)
CuCl2·2H2O (0.0340 g, 0.2 mmol) and HL (0.0775 g, 0.2 mmol) were mixed in 3 mL isopropanol and 3 mL methanol in a 25 mL Teflon-lined autoclave, heated at 80 °C for 3 days and then cooled to room temperature. Black strip crystals were obtained, the yield was 53.9%. Anal. calcd for C24H23ClCuN4O: C, 57.83; H, 4.65, N, 11.24. Found: C, 57.89; H, 4.63; N, 11.24. IR (KBr pellet, cm−1): 3412s, 1612s, 1527vs, 1483s, 1420s, 1352s, 1242m, 1202s, 1142s, 831w, 783w.Synthesis of [ZnL1(Ac)]·0.5CH3CH(OH)CH3 (2)
Zn(Ac)2·2H2O (0.0220 g, 0.1 mmol), 4-(diethylamino)salicylaldehyde (0.0386 g, 0.2 mmol) and 2-(2-aminophenyl)benzimidazole (0.0418 g, 0.2 mmol) were mixed with 5 mL isopropanol in a 25 mL Teflon-lined autoclave, heated at 80 °C for 3 days, then cooled to room temperature. Prismatic crystals were obtained, the yield was 83.9%. Anal. calcd for C27.5H30N4O3.5Zn: C, 61.40; H, 5.62, N, 10.41. Found: C, 61.24; H, 5.39; N, 10.32. IR (KBr pellet, cm−1): 3431s, 1604s, 1585vs, 1491s, 1385s, 1346s, 1256m, 1193m, 1141s, 1015w, 827w, 740w.X-ray crystallography
Single-crystal data were obtained from a Bruker APEX II CCD diffractometer with graphite monochromated Mo-Kα radiation (λ) at 293 K. The structure was solved by the direct method and refined by full-matrix least-squares built on F2 by service of the SHELX 97 program.16 Crystal data and structure refinement parameters for HL, 1 and 2 are tabulated in Table 1. The bond lengths and angles of HL, 1 and 2 are shown in Tables S1–S3,† respectively.| Compound | HL | 1 | 2 |
|---|---|---|---|
| a R1 = ∑(|Fo| − |Fc|)/|Fo|; wR2 = {∑[(w|Fo2| − |Fc2|)2/∑w(Fo2)2]}1/2. | |||
| Formula | C24H24N4O | C25H27ClCuN4O2 | C27.5H30N4O3.5Zn |
| Fw | 384.47 | 514.50 | 537.97 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n | Cc |
| a (Å) | 14.0046(13) | 8.089(3) | 20.646(2) |
| b (Å) | 8.9892(8) | 20.908(8) | 18.537(2) |
| c (Å) | 16.6000(15) | 13.943(5) | 15.4375(18) |
| α (°) | 90 | 90 | 90 |
| β (°) | 105.069(2) | 90.474(8) | 117.913(2) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 2017.9(3) | 2358.2(16) | 5220.7(10) |
| Z | 4 | 4 | 8 |
| Calc. dens. (g cm−3) | 1.266 | 1.499 | 1.369 |
| F(000) | 816 | 1068 | 2248 |
| Ref. collected/unique, Rint | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 382/4650, Rint = 0.0499 382/4650, Rint = 0.0499 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112/5458, Rint = 0.0783 112/5458, Rint = 0.0783 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 747/7163, Rint = 0.0613 747/7163, Rint = 0.0613 |
| Goodness-of-fit | 1.464 | 1.048 | 1.022 |
| R indices [I > 2σ(I)] | R1 = 0.0720 | R1 = 0.0626 | R1 = 0.0475 |
| wR2 = 0.2045 | wR2 = 0.1600 | wR2 = 0.0940 | |
Methods of cell imaging
DMEM was used to culture SH-SY5Y cell line. Cells were incubated with 20 μM CuCl2 at 37 °C for 8 h, washed with PBS three times in order to remove the remaining CuCl2, then incubated the cells with 20 μM HL for 30 min at room temperature. The incubated cells were smeared into a glass slide after washing with PBS. Fluorescent images of the cells were obtained from a confocal laser scanning microscope.Conclusions
A new quinazoline compound 6-(4-diethylamino)phenol-2-yl-(5,6-dihydrobenzimidazo[1,2-c])quinazoline (HL) was synthesized and can be used as a chemosensor for Cu(II) and Zn(II) in aqueous media. The selectivity of HL to Cu(II) and Zn(II) relies on the different excitation wavelengths. In the presence of Cu(II) ion fluorescence of HL was quenched because of ligand to metal charge transfer (LMCT) from L1 to Cu(II). The binding modes of HL with Cu(II) and Zn(II) were confirmed by both of spectroscopic data of HL with Cu(II) and Zn(II) ions in DMSO/H2O and the structures of [CuL1Cl] and [ZnL1(Ac)] complexes. Both Cu(II) and Zn(II) are coordinated with two nitrogen atoms and one oxygen atom from HL, forming Cu(II) and Zn(II) complexes. The results show that N–H and O–H group-containing quinazoline fluorophores can be used as platform for the detection of multi-metal ions.Notes and references
- (a) G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433 CrossRef CAS PubMed; (b) Z.-P. Liu, W.-J. He and Z.-J. Guo, Chem. Soc. Rev., 2013, 42, 1568 RSC.
- J.-L. Fan, P. Zhan, M.-M. Hu, W. Sun, J.-Z. Tang, J.-Y. Wang, S.-G. Sun, F.-L. Song and X.-J. Peng, Org. Lett., 2013, 15, 492 CrossRef CAS PubMed.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995 CrossRef CAS PubMed.
- (a) N. C. Lim, H. C. Freake and C. Bruckner, Chem.–Eur. J., 2005, 11, 38 CrossRef PubMed; (b) K. Tayade, S. K. Sahoo, B. Bondhopadhyay, V. K. Bhardwaj, N. Singh, A. Basu, R. Bendre and A. Kuwar, Biosens. Bioelectron., 2014, 61, 429 CrossRef CAS PubMed.
- P. Ozturk, E. Kurutas, A. Ataseven, N. Dokur, Y. Gumusalan, A. Gorur, L. Tamer and S. Inaloz, J. Trace Elem. Med. Biol., 2014, 28, 266 CAS.
- L. Marger, C. R. Schubert and D. Bertrand, Biochem. Pharmacol., 2014, 91, 426 CrossRef CAS PubMed.
- (a) G. J. Brewer, Biofactors, 2012, 38, 107 CrossRef CAS PubMed; (b) A. I. Bush, Trends Neurosci., 2003, 26, 207 CrossRef CAS PubMed; (c) A. B. Chausmer, J. Am. Coll. Nutr., 1998, 17, 109 CrossRef CAS PubMed.
- (a) K. Komatsu, K. Kikuchi, H. Kojima, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2005, 127, 10197 CrossRef CAS PubMed; (b) H. Woo, S. Cho, Y. Han, W.-S. Chae, D.-R. Ahn, Y. You and W. Nam, J. Am. Chem. Soc., 2013, 135, 4771 CrossRef CAS PubMed; (c) D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194 CrossRef CAS PubMed; (d) Y.-J. Zheng, J. Orbulescu, X.-J. Ji, F.-M. Andreopoulos, S. M. Pham and R. M. Leblanc, J. Am. Chem. Soc., 2003, 125, 2680 CrossRef CAS PubMed.
- (a) S. K. Kim, S. H. Lee, J. Y. Lee, R. A. Bartsch and J. S. Kim, J. Am. Chem. Soc., 2004, 126, 16499 CrossRef CAS PubMed; (b) M. Schmittel and H.-W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893 CrossRef CAS PubMed; (c) J.-L. Wang, W.-Y. Lin and W.-L. Li, Chem.–Eur. J., 2012, 18, 13629 CrossRef CAS PubMed; (d) K. P. Divya, S. Savithri and A. Ajayaghosh, Chem. Commun., 2014, 50, 6020 RSC; (e) W. Cao, X.-J. Zheng, J.-P. Sun, W.-T. Wong, D.-C. Fang, J.-X. Zhang and L.-P. Jin, Inorg. Chem., 2014, 53, 3012 CrossRef CAS PubMed.
- (a) S. Anbu, R. Ravishankaran, M. F. C. Guedes Da Silva, A. A. Karande and A. J. L. Pombeiro, Inorg. Chem., 2014, 53, 6655 CrossRef CAS PubMed; (b) Z.-X. Li, L.-F. Zhang, L.-N. Wang, Y.-K. Guo, L.-H. Cai, M.-M. Yu and L.-H. Wei, Chem. Commun., 2011, 47, 5798 RSC; (c) S.-J. Xia, G. Liu and S.-Z. Pu, J. Mater. Chem. C, 2015, 3, 4023 RSC; (d) M.-M. Yu, Z.-X. Li, L.-H. Wei, D.-H. Wei and M.-S. Tang, Org. Lett., 2008, 10, 5115 CrossRef CAS PubMed; (e) D. Sarkar, A. K. Pramanik and T. K. Mondal, RSC Adv., 2015, 5, 7647 RSC; (f) P. Wang, L.-X. Liu, P.-P. Zhou, W.-Y. Wu, J. Wu, W.-S. Liu and Y. Tang, Biosens. Bioelectron., 2015, 72, 80 CrossRef CAS PubMed.
- (a) U. C. Saha, B. Chattopadhyay, K. Dhara, S. K. Mandal, S. Sarkar, A. R. Khuda-Bukhsh, M. Mukherjee, M. Helliwell and P. Chattopadhyay, Inorg. Chem., 2011, 50, 1213 CrossRef CAS PubMed; (b) R. Pandey, R. K. Gupta, M. Shahid, B. Maiti, A. Misra and D. S. Pandey, Inorg. Chem., 2012, 51, 298 CrossRef CAS PubMed; (c) M. Mukherjee, B. Sen, S. Pal, M. S. Hundal, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, RSC Adv., 2013, 3, 19978 RSC; (d) D. Jehyanthi, M. Iniya, K. Krishnaveni and D. Chellapa, RSC Adv., 2013, 3, 20984 RSC; (e) A. Kumar, R. Pandey, A. Kumar and D. S. Pandey, RSC Adv., 2014, 4, 55967 RSC; (f) S. Mukhopadhyay, R. K. Gupta, A. Biswas, A. Kumar, M. Dubey, M. S. Hundal and D. S. Pandey, Dalton Trans., 2015, 44, 7118 RSC; (g) Y. Liu, D. Wang, X.-J. Zheng and L.-P. Jin, RSC Adv., 2015, 5, 36987 RSC.
- (a) W. Cao, X.-J. Zheng, D.-C. Fang and L.-P. Jin, Dalton Trans., 2014, 43, 7298 RSC; (b) W. Cao, X.-J. Zheng, D.-C. Fang and L.-P. Jin, Dalton Trans., 2015, 44, 5191 RSC; (c) L.-J. Tang and M.-J. Cai, Sens. Actuators, B, 2012, 173, 862 CrossRef CAS.
- S. Jana, S. Dalapati and N. Guchhait, J. Phys. Chem. A, 2012, 116, 10948 CrossRef CAS PubMed.
- P. Wang, L.-X. Liu, P.-P. Zhou, W.-Y. Wu, J. Wu, W.-S. Liu and Y. Tang, Biosens. Bioelectron., 2015, 72, 80 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- G. M. Sheldrick, in SHELXS-97, Program for solution of crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis spectrum, ESI and intramolecular and intermolecular hydrogen bonds of HL; the excitation and fluorescence spectra of HL (10 μM) + 1 equiv. Zn(II); competition experiments of HL with Cu2+ and Zn2+. In the presence of various metal ions; UV-vis spectrum of Zn(II) complex, HL (10 μM) + 1 equiv. Zn(II) and Cu(II) complex, HL (10 μM) + 1 equiv. Cu(II); Benesi–Hildebrand plot and normalized response of HL with Cu2+ and Zn2+; bond distances and angles for HL, [CuL1Cl] and [ZnL1(Ac)]. CCDC 1425680–1425682. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra01030j |
| This journal is © The Royal Society of Chemistry 2016 |