Mesoporous MgO·Al2O3 nanopowder-supported meso–macroporous nickel catalysts: a new path to high-performance biogas reforming for syngas
Narges Habibia,
Hamidreza Arandiyanb and
Mehran Rezaei*ac
aCatalyst and Advanced Materials Research Laboratory, Chemical Engineering Department, Faculty of Engineering, University of Kashan, Kashan, Iran
bParticles and Catalysis Research Group, School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
cInstitute of Nanoscience and Nanotechnology, University of Kashan, Kashan, Iran. E-mail: rezaei@kashanu.ac.ir
First published on 16th March 2016
Abstract
Mesoporous nanocrystalline MgO·Al2O3 powders with different MgO/Al2O3 molar ratios were synthesized by a new and simple sol–gel route using C3H6O (propylene oxide) as a gelation agent. The prepared powders were employed as a support for preparation of 10 wt% Ni catalysts in biogas reforming, for the production of synthesis gas. This simple sol–gel method led to the preparation of powders with high BET surface area in the range of 252.8–301.6 m2 g−1 depending on the MgO/Al2O3 molar ratio after calcination at 700 °C. The samples also exhibited narrow single modal pore size distributions in the mesopore region. The H2-TPR analysis revealed that increasing the MgO/Al2O3 molar ratio shifted the Tmax of the reduction peaks to higher temperature, indicating the lower reducibility of the prepared catalysts with high MgO content. The NH3-TPD also confirmed the increase in basicity of the prepared samples with increasing MgO/Al2O3 molar ratio. The prepared catalysts exhibited high potential as catalysts for biogas reforming with high stability, and the catalysts with higher content of MgO showed higher resistance against carbon formation.
Introduction
Decreasing the energy reserves based on fossil fuels and concerns about the environmental regulations for CO2 emissions cause the consideration of renewable resources for syngas (a mixture of H2 and CO) production as an essential feedstock for the production of synfuels, methanol, DME, higher alcohols, etc.1–4 In recent years, biogas, a mixture of CH4 (50–70%) and CO2 (30–50%) with small amounts of impurities such as NH3, H2S, organic sulfur and water vapor, has been considered as a renewable resource of carbon for synthesis gas production.5–7 The biogas after purification can be transformed to synthesis gas by reforming process, either with or without steam addition, depending on the molar H2/CO ratio in the reformed biogas. Biogas reforming without addition of steam (eqn (1)), is an environmentally friendly because synthesis gas is being produced from carbon dioxide (CO2) and methane (CH4), which are both greenhouse gases.8,9| CH4 + CO2 ↔ 2CO + 2H2, ΔH0298 K = +247 kJ mol−1 | (1) |
The transition metals (group VIII of the periodic table) and noble metals are active catalysts for biogas reforming (dry reforming) process.8–12 Among these metals, Ru and Rh are the most active metals and Ni, Ir, Pt, Pd and Co, can also be considered as active catalysts.12 It is known that the main challenge in carbon dioxide reforming is carbon formation on the catalyst surface, which causes the catalyst deactivation.8,9,12,13 There are two pathways for carbon formation in dry reforming of methane: decomposition of methane (eqn (2)) and the disproportion reaction of CO (eqn (3)).
| CH4 → C + 2H2, ΔH0298 K = +75 kJ mol−1 | (2) |
| 2CO → C + CO2, ΔH0298 K = −172 kJ mol−1 | (3) |
The rate of carbon formation, catalytic activity and stability are known to depend on the active metal dispersion, crystallite size, strong metal–support interaction (SMSI) and surface acidity/basicity of mixed oxides.14–17 The noble metal catalysts exhibit a lower degree of carbon formation compared to Ni- and Co-based catalysts. However, the lower price is an attractive point for the use of Ni- and Co-based catalysts, mainly Ni-catalysts in dry reforming. Although Ni based catalysts exhibit higher degree of carbon formation, the high resistance to carbon formation can be obtained by the use of a promoter, changing the support, or by optimizing the catalyst's preparation.16 A careful choice of a suitable support is crucial. Since the dry reforming reaction involves the adsorption and dissociation of acidic CO2, basic promoters and supports like MgO can enhance the ability of CO2 chemisorption, which increases the coke resistance of the catalyst.18 Among the catalyst supports, alumina is the most conventional support for reforming catalysts, although other supports such as MgO, MgAl2O4, CaAl2O4 are also employed as catalyst support.8,9,13,17 Addition of MgO to Al2O3 can increase the basic properties of the catalyst support and consequently increase the carbon resistance and stability of the nickel based catalyst. As mentioned before, the preparation conditions can affect the textural and catalytic performance of the catalyst. There are several methods for preparation of catalyst supports, such as precipitation, sol–gel, solid state reaction, etc.19–22
Herein, we demonstrate for the first time the preparation and catalytic biogas reforming for syngas production of MgO·Al2O3 mixed metal oxide nanopowders with different MgO/Al2O3 molar ratios by employing propylene oxide as gelation agent by the novel surfactant free self-assembly (NSFS) sol–gel method. The prepared mixed metal oxide nanopowders were employed as catalyst support for preparation of nickel catalysts in biogas reforming reaction.
Experimental section
Materials
The starting materials were Al(NO3)3·9H2O and Mg(NO3)2·6H2O, Ni(NO3)2·6H2O as Al, Mg and Ni precursors. Absolute ethanol (C2H5OH, Merck) and propylene oxide (C3H6O, Merck) were also used as solvent and gelation agent, respectively. All the reagents were used without further purification.Sample preparation
For preparation of the samples with different MgO/Al2O3 molar ratios, desired amounts of Al(NO3)3·9H2O and Mg(NO3)2·6H2O (Mg and Al precursors) were dissolved in specific content of absolute ethanol (C2H5OH/(Al3+ + Mg2+) = 40) under vigorous magnetic stirring at room temperature. After that the propylene oxide (propylene oxide/(Al3+ + Mg2+) molar ratio = 11) was then added to the prepared solution containing metal precursors. After addition of propylene oxide, an exothermic reaction took place, accompanied by a gel formation within several minutes. After obtaining a uniform gel formation, the resulting gel was aged at room temperature for 30 min and then dried at 85 °C for 24 h. The dried gel was calcined at 700 °C with a ramp rate of 3 °C min−1 for 3 h in air atmosphere. Finally, the catalysts with 10 wt% Ni were prepared by the wet impregnation of the prepared powders with different MgO/Al2O3 molar ratios using an aqueous solution of nickel nitrate with appropriate concentration at room temperature. After impregnation, the samples were dried at 85 °C for 24 h and calcined at 500 °C for 4 h.Characterization
The crystalline structure and the phases present in the prepared samples were evaluated by X-ray diffraction (XRD) analysis using a PANalytical X'Pert-Pro diffractometer in a scanning range 2θ = 10–80°. The pore size, pore volume, specific surface area and pore size distributions were determined by nitrogen adsorption/desorption analysis using Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods at boiling temperature of liquid nitrogen (−196 °C) by a BELSORP-mini II analyzer. The reduction behavior and the effect of catalyst composition on the reduction temperature of the prepared samples were investigated by temperature programmed reduction (TPR) analysis using a Micromeritics chemisorb 2750 equipment. In this analysis, 100 mg calcined sample was degassed under an argon atmosphere at 200 °C for 1 h. After that, a gaseous stream containing a mixture of H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar (10
Ar (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) with a flow rate of 25 mL min−1 was passed over the sample and the temperature increased with a ramp rate of 10 °C min−1. The amount of H2 consumption during the reduction was measured using a thermal conductivity detector (TCD). The resistance of catalysts against carbon formation and also the type of carbon deposited over the spent catalysts was studied by oxidation and hydrogenation of deposited carbon by the temperature-programmed oxidation and hydrogenation (TPO and TPH) techniques using a similar apparatus as described for TPR analysis by introducing an oxidizing gas flow (30 mL min−1, a mixture of O2
90) with a flow rate of 25 mL min−1 was passed over the sample and the temperature increased with a ramp rate of 10 °C min−1. The amount of H2 consumption during the reduction was measured using a thermal conductivity detector (TCD). The resistance of catalysts against carbon formation and also the type of carbon deposited over the spent catalysts was studied by oxidation and hydrogenation of deposited carbon by the temperature-programmed oxidation and hydrogenation (TPO and TPH) techniques using a similar apparatus as described for TPR analysis by introducing an oxidizing gas flow (30 mL min−1, a mixture of O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He (5
He (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95)) for TPO and a gas flow (30 mL min−1) containing a mixture of H2
95)) for TPO and a gas flow (30 mL min−1) containing a mixture of H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar (10
Ar (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) for TPH analysis. The acidic properties of the samples with different compositions were determined by the desorption of adsorbed ammonia on the samples using a temperature programmed desorption technique (NH3-TPD) in a quartz fixed bed micro reactor using the same apparatus as mentioned for TPR analysis. In NH3-TPD experiments, the sample (0.2 g) was degassed at 250 °C for 1 h under nitrogen stream and after cooled down to 50 °C, the degassed sample was saturated in a NH3 stream for 30 min. After this step the saturated sample was purged with He (20 mL min−1) at 50 °C for 30 min to remove the physically adsorbed NH3. NH3-TPD was carried out with a ramp of 10 °C min−1 from room temperature to a needed temperature under He stream. A FTIR Bruker-Tensor 270 spectrometer was used for the Fourier transform infrared spectroscopy (FTIR) analysis. The morphology of the prepared samples and also the carbon deposited over the spent catalysts was studied by a scanning electron microscope (SEM, Nova NanoSEM 650).
90) for TPH analysis. The acidic properties of the samples with different compositions were determined by the desorption of adsorbed ammonia on the samples using a temperature programmed desorption technique (NH3-TPD) in a quartz fixed bed micro reactor using the same apparatus as mentioned for TPR analysis. In NH3-TPD experiments, the sample (0.2 g) was degassed at 250 °C for 1 h under nitrogen stream and after cooled down to 50 °C, the degassed sample was saturated in a NH3 stream for 30 min. After this step the saturated sample was purged with He (20 mL min−1) at 50 °C for 30 min to remove the physically adsorbed NH3. NH3-TPD was carried out with a ramp of 10 °C min−1 from room temperature to a needed temperature under He stream. A FTIR Bruker-Tensor 270 spectrometer was used for the Fourier transform infrared spectroscopy (FTIR) analysis. The morphology of the prepared samples and also the carbon deposited over the spent catalysts was studied by a scanning electron microscope (SEM, Nova NanoSEM 650).
Catalytic evaluation
A vertical quartz microreactor with an inner diameter of 7.0 mm was used for evaluating the catalytic performance of the prepared catalysts at atmospheric pressure. Prior to reaction, the calcined samples were pressed, crushed and sieved and the catalyst particles (0.25–0.5 mm) were reduced in situ at 700 °C for 3 h in H2 stream (30 mL min−1). After reduction, the temperature of the catalyst bed was set to 550 °C and then the feed gas consisting of a mixture of CH4 and CO2 with desired flow and ratio was introduced into the reactor and the catalytic performance was determined at different temperatures. The gas composition of reactants and products were analyzed using an online gas chromatograph (Young Lin) equipped with a TCD detector and a Carboxen 1010 column.Results and discussion
Among the methods for preparation of mixed metal oxides, the sol–gel process shows high potential with a number of benefits, such as high chemical homogeneity, high purity, low calcination temperatures, etc.23 In conventional sol–gel method, the metal alkoxides were used, which form a sol of metal oxide nanoparticles due to hydrolysis and condensation processes.24,25 This sol–gel technique has some advantages such as easiness and high efficiency for synthesis of metal oxides with high porosity. However, most of the metal alkoxides are expensive, not commercially available, sensitive to light, heat and water vapor, which make their application difficult in this method. Therefore, studies on the development of non-alkoxide sol–gel method are of great importance. One of this non-alkoxide sol–gel methods is based on the usage of an epoxide as a gelation agent.26,27 This method needs lower steps compared to other conventional methods and can be considered as a low temperature and low cost synthesis method for preparation of metal oxides. The epoxides as cyclic ethers with higher reactivity compared to simple ethers and can act as an acid scavenger via protonation of the epoxide oxygen and subsequent ring opening by the nucleophilic anionic conjugate base, as shown in eqn (4).28,29
 | (4) |
 | (5) |
The hydrated aluminum ion ([Al(H2O)6]3+) formed by dissolving of aluminum nitrate in ethanol can be considered as a strong acid, which can protonate the epoxides. According to eqn (5), C3H6O consumes protons from the [Al(H2O)6]3+ and generates the Al(OH)(H2O)52+ species, which can perform hydrolysis and condensation reaction to form more condensed Al(III) oxide species as illustrated in eqn (6). The epoxide consumed the generated protons in these reactions. The nucleophilic attack of nitrate ions in the solution to protonated epoxide can be caused an irreversible ring-opening as shown in eqn (7). This reaction eliminates the protons from the solution and causes the further hydrolysis of Al(III) complex, which leads to gel formation.
| 2Al(OH)(H2O)52+ ⇌ [(H2O)5Al–O–2Al(H2O)5]4+ + H2O | (6) |
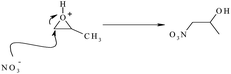 | (7) |
Fig. 1 shows the FTIR spectra of the as-prepared and calcined samples with MgO/Al2O3 molar ratio of 1. The observed peaks in dried sample at around 3400 cm−1 and 1630 cm−1 are related to the broad H–O–H stretching and OH bending vibration of water, respectively. It is seen that the intensity of these peaks decreased after calcination. The appeared bands in these two regions in the spectrum of the calcined sample are related to adsorbed water from the atmosphere. Moreover, the strong absorption band at 1382 cm−1 can be assigned to NO3− characteristic vibration. This band was almost disappeared after calcination due to thermal decomposition of nitrate groups. The two peaks located at 2360 cm−1 and 2340 cm−1 in both samples come from the atmospheric CO2. The band at 2420 cm−1 is due to the bending vibration of CH3, which disappeared after calcination.
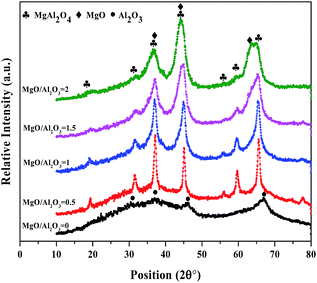
The XRD patterns of the samples with different MgO/Al2O3 molar ratios are shown in Fig. 2. The diffraction peaks of pure Al2O3 can be assigned to γ-alumina (JCPDS card 00-029-0063) phase. The low degree of crystallinity of this sample is due to small crystallite size. Addition of MgO to Al2O3 increased the crystallinity of the samples. For the samples with different MgO/Al2O3 molar ratios, different crystalline phases (MgAl2O4, MgO, Al2O3) were observed. The width of the diffraction peaks located around 37, 44 and 63° increased by increasing in MgO/Al2O3 molar ratio and the shoulder peak appeared on the diffraction peak around 63° clearly confirmed the existence of MgO crystalline phase. In addition, the intensity of the diffraction peaks related to MgAl2O4 spinel became weaker with increasing in MgO/Al2O3 molar ratio, indicating of the decrease in the content of MgAl2O4.
The porous structures of the prepared samples with different MgO/Al2O3 molar ratios were further substantiated by the pore size and N2 adsorption–desorption isotherms in Fig. 3a and b, respectively. As can be seen, all the samples exhibited a narrow pore size distribution in mesopore framework.
 | ||
| Fig. 3 (a) Pore size distributions and (b) N2 adsorption/desorption isotherms of the prepared samples with different MgO/Al2O3 molar ratios. | ||
The pore size distribution is affected by the MgO/Al2O3 molar ratio and increasing in MgO/Al2O3 molar ratio shifted the pore size distributions to larger sizes. The N2 adsorption/desorption isotherms of the prepared samples are also affected by the MgO/Al2O3 molar ratio. Each of the samples displayed a type IV isotherm. Type IV isotherms are typical for mesoporous materials. Important characteristics are the increase in volume adsorbed at higher p/p0 caused by adsorption in mesopores as well as a hysteresis loop. A distinct increase in adsorbed nitrogen in the low p/p0 region (lower than 0.05) in type IV isotherms indicates the existence of micropores associated with mesopores. The increase in adsorbed nitrogen volume at higher p/p0 in type IV isotherms is due to capillary condensation below the expected condensation pressure of the nitrogen.
According to IUPAC classification, all the samples showed H2 type of hysteresis loop. This type of hysteresis is observed in the case of materials having mesopores structure in intercommunication and almost of triangular shape and is typically due to pores which are interconnected, often with smaller entrances than bodies. In such cases, one often refers to ink bottle-shaped pores. It is seen that the formation point and also the hysteresis loop were shifted to higher relative pressures by increasing in MgO/Al2O3 molar ratio, which is due to the increase in mesopore width. In addition, the amount of volume adsorbed/desorbed is higher for the samples with higher MgO/Al2O3 molar ratios due to higher pore volume, and the results are presented in Table 1.
| MgO/Al2O3 | BET (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|
| 0 | 301.6 | 0.327 | 4.33 |
| 0.5 | 282.2 | 0.336 | 4.76 |
| 1 | 264 | 0.436 | 6.61 |
| 1.5 | 261.4 | 0.622 | 9.53 |
| 2 | 252.8 | 0.618 | 9.78 |
The structural properties of the prepared samples are reported in Table 1. As can be seen, increasing in MgO/Al2O3 molar ratio decreased the specific surface area and also increased both the pore volume and pore size. It is seen that the pure Al2O3 exhibited a high specific surface area at 700 °C. The XRD patterns of the catalysts with different MgO/Al2O3 molar ratios are shown in Fig. 4. The NiO diffraction peaks clearly were observed on the 10%Ni/Al2O3 and 10%Ni/2Al2O3·MgO samples. As can be seen, the intensity of the NiO diffraction peaks in 10%Ni/2Al2O3·MgO was higher than those observed in 10%Ni/Al2O3 due to its lower specific surface area. Therefore, it can be concluded that NiO dispersion was higher on the 10%Ni/Al2O3 catalyst compared to 10%Ni/2Al2O3·MgO. For MgO, the peak positions are at 2θ = 42.8, 62.23, and 74.59°, respectively.
For NiO, the corresponding peaks are at 2θ = 43.4, 62.94, 75.43°, respectively. The above three diffraction lines can be used to identify the possibility of formation of a solid solution. Fig. 4 illustrated that the diffraction peaks related to NiO were not observed due to formation of NiO–MgO solid solution or peak overlapping between NiO and MgAl2O4. The structural properties of the prepared catalysts with different MgO/Al2O3 molar ratios are presented in Table 2. It is clearly seen that the catalysts exhibited the lower specific surface area compared to catalyst supports, which might be attributed to partial blockage of the pores of the catalyst support by nickel oxide clusters. Additionally, increasing in MgO/Al2O3 molar ratio in the catalysts decreased the specific surface area and increased both the pore volume and pore size of the catalysts.
| Catalyst | BET (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | |||
|---|---|---|---|---|---|---|
| Calcined | Spent | Calcined | Spent | Calcined | Spent | |
| 10Ni/Al2O3 | 258.2 | 136.3 | 0.355 | 0.321 | 5.51 | 9.42 |
| 10Ni/2Al2O3·MgO | 236.3 | 130.9 | 0.362 | 0.336 | 6.13 | 10.28 |
| 10Ni/Al2O3·MgO | 224.2 | 120.4 | 0.391 | 0.358 | 6.97 | 11.89 |
| 10Ni/0.66Al2O3·MgO | 216.3 | 145.1 | 0.412 | 0.385 | 7.62 | 10.626 |
| 10Ni/0.5Al2O3·MgO | 220.5 | 152.4 | 0.457 | 0.413 | 8.30 | 10.84 |
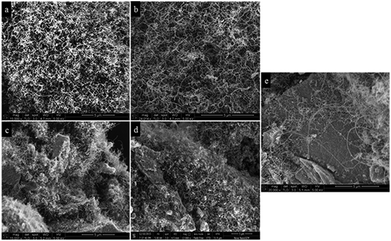
The pore size distributions and N2 adsorption/desorption isotherms of the prepared catalysts are shown in Fig. 5a and b, respectively. Increasing in MgO/Al2O3 molar ratio shifted the pore size distribution to larger sizes. All catalysts exhibited mesoporous structure with a narrow pore size distribution smaller than 4 nm. Meanwhile, the appearance of a IV type isotherm with H2 shaped hysteresis loop in the p/p0 range of 0.2–0.9 of each sample was an indication of mesoporous formation. The SEM images of the calcined catalysts with different MgO/Al2O3 molar ratios are shown in Fig. 6. It is seen that the samples showed petal-like structures with many cavities (flower like) which consists of mesoporous uniformly indicated on the support (Al2O3 and Al2O3·MgO). It seems that the size of the cavities on the 10Ni/Al2O3 sample is larger than those observed on the other samples. The flower-like samples are assembled by 2D thin nanosheets with average thickness ∼20 nm. Specifically for sample 10Ni/0.5Al2O3·MgO the surface of the nanosheets becomes coarse and porous, suggesting a highly porous texture (Fig. 6e). Thus, a novel technique of NSFS sol–gel method provides a simple approach to synthesize a robust and unique structure.
 | ||
| Fig. 6 SEM analysis of the calcined catalysts, (a and b) 10Ni/Al2O3, (c and d) 10Ni/2Al2O3·MgO, (e and f) 10Ni/Al2O3·MgO, (g and h) 10Ni/0.66Al2O3·MgO and (i and j) 10Ni/0.5Al2O3·MgO. | ||
The H2-TPR profiles of the prepared catalysts with different MgO/Al2O3 molar ratios are illustrated in Fig. 7a. In H2-TPR profile of the 10%Ni/Al2O3 catalyst four reduction peaks were observed at different temperatures. The first low temperature reduction peak around 400 °C was related to reduction of bulk nickel oxide. The second reduction peak at 460 °C was assigned to reduction of nickel oxide with higher interaction with catalyst support. The reduction peak at 700 °C was related to nickel oxide with strong interaction with Al2O3 and the shoulder appeared at around 800 °C was due to reduction of nickel aluminate. It is seen that the addition of MgO to Al2O3 shifted the Tmax of the main reduction peak to higher temperature. In addition the intensity of the low temperature peaks decreased due to increasing in metal–support interaction. As can be seen, increasing in MgO/Al2O3 molar ratio significantly affected the reduction behavior of the nickel catalyst and shifted the reduction temperature of nickel oxide to higher temperatures. The last reduction peaks observed at temperatures higher than 750 °C in catalysts containing MgO is ascribed to the reduction of Ni2+ ions located in the subsurface layers of the MgO lattice, showing a stronger interaction between NiO and MgO and formation of NiO–MgO solid solution. The increase in reduction temperature of the nickel catalysts containing the MgO is attributed to the formation of a solid solution of NiO in MgO. On the reduction of NiO–MgO solid solution, the outermost Ni0 atoms nucleate to form fine metal particles. However, the nickel which is deeper remain isolated in the matrix of MgO as Ni0 or as a charged Ni species in a low oxidation state. The reduced NiO–MgO solid solution can provide a strong ionic environment at the support–metal particle interface and even more so for the reduced species (Ni0), which are at the surface but not fully exposed.17
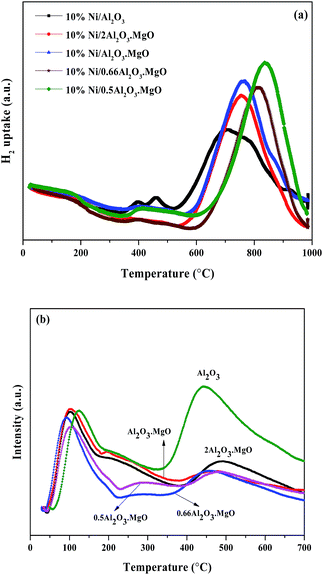 | ||
| Fig. 7 (a) TPR analysis of the catalysts and (b) NH3-TPD of the samples with different MgO/Al2O3 molar ratios. | ||
The acidic properties of the prepared samples were evaluated by NH3-TPD and their profiles are illustrated in Fig. 7b. In the TPD profile of the pure Al2O3 two main desorption peaks were observed at different temperatures. The first low temperature peak at 125 °C is related to weak acidic sites and the second peak appeared at 420 °C is assigned to strong acidic sites over the Al2O3 surface. Addition of MgO to Al2O3 decreased the intensity and also the area of the desorption peaks, indicating the lower acidic properties of the samples containing MgO. It is seen that increasing in MgO/Al2O3 molar ratio also enhanced the basic properties of the samples and decreased the amount of the desorbed ammonia from the solid surface.
The catalytic performances of the prepared catalysts are shown in Fig. 8. As can be seen, all catalysts exhibited high catalytic performance in dry reforming reaction. The methane and carbon dioxide conversions increased by increasing reaction temperature due to endothermic nature of carbon dioxide reforming of methane. Although the CH4 and CO2 conversions of the catalysts are close together, however the 10%Ni/0.66Al2O3·MgO catalyst exhibited the highest activity. In addition, the CO2 conversion is higher than the CH4 conversion (Fig. 8b) due to occurrence of reverse water gas shift reaction. In addition, the prepared catalysts possessed high stability without any decrease in methane conversion during 5 h time on stream, Fig. 8c. The results also showed a stable H2 and CO selectivities for the prepared catalysts during the reaction and as can be seen, the CO selectivity is higher than H2 selectivity due to occurrence of reverse water gas shift reaction, Fig. 8d.
 | ||
Fig. 8 (a) CH4 conversion, (b) CO2 conversion, (c) stability of the catalysts at 700 °C and (d) H2 and CO selectivities at 700 °C, reaction conditions: GHSV = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, CH4 000 h−1, CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 = 1. CO2 = 1. | ||
The TPO analysis of the spent catalysts is shown in Fig. 9a. It is seen that one oxidation peak was observed in TPO profile of the spent catalysts. As can be seen, for 10 wt%Ni/Al2O3 and 10 wt%Ni/2Al2O3·MgO catalysts the sharp increase in TPO profile at 700 °C is related to fast burning of carbon (whisker type) deposited over the surface of the catalysts. The fast burning of carbon due to large amounts of carbon deposited over the catalyst surface releases a large volume of gaseous products during the TPO analysis, which causes an intense change in intensity measured by TCD detector. The 10 wt%Ni/Al2O3 catalyst exhibited the highest degree of carbon formation. Addition of MgO to Al2O3 decreased the amount of the deposited carbon, since the area of the peaks observed in TPO profile decreased by addition of MgO. Furthermore, increasing in MgO/Al2O3 molar ratio decreased the amount of deposited carbon, which is due to higher basicity of catalyst with higher MgO contents (Fig. 7b). It has generally been accepted that the CO2 has acidic properties. Increasing in basicity of the catalyst improves the adsorption of CO2 and suppresses the carbon deposition over the catalyst surface by inhibiting the Boudouard reaction.
 | ||
| Fig. 9 (a) TPO and (b) TPH profiles of the spent catalysts, (1) 10Ni/Al2O3, (2) 10Ni/2Al2O3·MgO, (3) 10Ni/Al2O3·MgO, (4) 10Ni/0.66Al2O3·MgO and (5) 10Ni/0.5Al2O3·MgO. | ||
TPH analysis was performed to investigate the type of carbon deposited over the spent catalysts. The results are shown in Fig. 9b. These results confirmed the formation of different carbon species with different reactivities in hydrogenation reaction. The first peaks observed at temperatures lower than 300 °C were related to removal of active carbon species such as CHx (x = 1, 2, 3).30 The second peaks observed in the TPH profiles at around 550 °C were related to whisker-type carbon,31 as observed in the SEM images. It is interesting to see the 10 wt%Ni/Al2O3 only exhibited the whisker type carbon an no active carbon species observed over this catalyst after reaction. However, for other catalysts both types of carbon were observed. As can be seen, addition of MgO to the catalyst decreased the amount of whisker carbon and increased the active carbon species over the catalyst. It is noted that whisker carbon is a filamentous carbon with a Ni crystallite at the end. It does not block active sites (for example, on Ni), but grows very rapidly between catalyst particles thus crushing them and finally plugging the reactor.
Under dry reforming conditions, nickel surfaces are covered with various CHx species. Without a fast and effective reaction to convert these species to CO and H2, these CHx species adsorbed on Ni surface can undergo further dehydrogenation, polymerization and rearrangement into highly stable carbon species. These stable carbon species not only show low reactivity in hydrogenation reaction as shown in TPH profiles, but also my dissolve into or encapsulate the nickel particles. Addition of MgO to catalyst support enhances the basic properties of the catalysts and improves the adsorption of CO2 during the dry reforming reaction which supplies the surface oxygen to prevent the coke deposition. The adsorbed CO2 can react with CHx species adsorbed on Ni surface and prevent the further dehydrogenation of CHx species and formation of low active carbon.
The SEM analysis of the spent catalysts is shown in Fig. 10. The analysis clearly showed the deposition of whisker carbon over the spent catalysts. It is seen that the amount of whisker carbon decreased with increasing in MgO/Al2O3 molar ratio, which is in agreement with the results obtained by TPO and TPH analyses.
 | ||
| Fig. 10 SEM images of the spent catalysts, (a) 10Ni/Al2O3, (b) 10Ni/2Al2O3·MgO, (c) 10Ni/Al2O3·MgO, (d) 10Ni/0.66Al2O3·MgO and (e) 10Ni/0.5Al2O3·MgO. | ||
Min et al.32 prepared the Ni–MgO–Al2O3 catalysts with different Mg/Al ratios by sol–gel method. They showed that in the variation of Mg/Al ratios, high coke resistance was obtained with increasing MgO loading, while high catalytic activity was observed with the catalysts of medium MgO/(MgO + Al2O3) ratio (0.44–0.86), of which the superior catalytic activity is likely attributed to high specific surface area and well dispersed Ni particles. They also showed that the Ni/Al2O3 catalyst exhibited a low catalytic stability in dry reforming reaction due to high amount of deposited carbon. They showed that the addition of MgO to the Ni–Al2O3 increases the interaction between NiO and support, as well as the rise of basicity which is beneficial for the adsorption of CO2.
Rong-jun et al.33 also showed that the catalytic activity of NiO/Al2O3 is also very low and a quick deactivation is also observed for the NiO/Al2O3 catalyst, as the acidic sites on the Al2O3 surface might lead to a serious coke deposition.
The pore size distributions and N2 adsorption/desorption isotherms of the spent catalysts are shown in Fig. 11a and b, respectively. It is seen that the mesoporosity was remained after reaction and the pore size distribution shifted to larger sizes compared to fresh catalysts. The N2 adsorption/desorption isotherms are also type IV with H2 hysteresis loop.
 | ||
| Fig. 11 (a) Pore size distributions and (b) N2 adsorption desorption isotherms of the spent catalysts. | ||
In addition, the specific surface area of the catalysts after reaction decreased due to sintering of the catalyst at high temperature and also the formation of the carbon over the catalyst surface. It is seen that the pore size also increased after reaction. The highest decrease in the BET surface area was observed for the 10Ni/Al2O3 and addition of MgO to the catalyst improved the thermal stability of the prepared catalysts and the highest thermal stability was observed for 10Ni/0.5Al2O3·MgO.
The effect of GHSV on the CH4 and CO2 conversions at 700 °C and CH4/CO2 molar ratio of one is shown in Fig. 12a. Increasing in GHSV decreased both the CH4 and CO2 conversions. The negative effect of high space velocity is probably caused by the decreased contact time between reactants and active centers. The effect of feed ratio on the catalytic performance is shown in Fig. 12b. The increase in CO2/CH4 molar ratio increased the methane conversion and decreased the CO2 conversion, which could be due to the excess amount of CO2 in the feed stream. In addition, the H2/CO molar ratio decreased by increasing in CO2/CH4 molar ratio due to more occurrence of reverse water gas shift reaction.
 | ||
| Fig. 12 Effect of (a) GHSV and (b) feed ratio on the catalytic performance of 10Ni/0.66Al2O3·MgO at 700 °C. | ||
Conclusions
A novel surfactant free self-assembly (NSFS) sol–gel method was successfully employed for synthesis of mesoporous nanostructured Al2O3·MgO powders with different MgO/Al2O3 molar ratios. The prepared samples possessed high specific surface area with narrow single modal pore size distribution. Increasing in MgO/Al2O3 molar ratio decreased the specific surface area and increased the basicity of the samples. The prepared powders were employed as support for preparation of 10 wt% Ni. The catalytic performance of the catalysts was investigated in biogas reforming. The results showed that the catalysts with higher MgO contents exhibited a lower degree of reducibility and the Tmax of reduction temperature shifted to higher temperatures with increasing in MgO/Al2O3 molar ratios. The catalytic results revealed that the prepared catalysts showed high catalytic activity and stability in biogas reforming. However, the 10 wt%Ni/0.66Al2O3·MgO exhibited the highest methane conversion. The TPO analysis showed that increasing in MgO/Al2O3 molar ratio decreased the amount of deposited carbon and TPH analysis confirmed the formation of whisker type carbon as the main type of deposited carbon on the spent catalysts. The SEM analysis also confirmed the results obtained by TPO and TPH analyses.Acknowledgements
The authors gratefully acknowledge the support from Iran National Science Foundation (INSF) and University of Kashan for supporting this work by Grant No. 158426/95.References
- D. Pakhare and J. Spivey, Chem. Soc. Rev., 2014, 43, 7813–7837 RSC.
- J. Chen, H. Arandiyan, X. Gao and J. Li, Catal. Surv. Asia, 2015, 19, 140–171 CrossRef CAS.
- H. R. Arandiyan and M. Parvari, J. Nat. Gas Chem., 2008, 17, 213–224 CrossRef CAS.
- W. Li, Z. Zhao, P. Ren and G. Wang, RSC Adv., 2015, 5, 100865–100872 RSC.
- H. Arandiyan, H. Chang, C. Liu, Y. Peng and J. Li, J. Mol. Catal. A: Chem., 2013, 378, 299–306 CrossRef CAS.
- L. Yao, J. Shi and C. Hu, RSC Adv., 2015, 5, 90168–90177 RSC.
- L. Zhang and Y. Zhang, RSC Adv., 2015, 5, 62173–62178 RSC.
- F. Meshkani and M. Rezaei, Int. J. Hydrogen Energy, 2010, 35, 10295–10301 CrossRef CAS.
- A. Ranjbar and M. Rezaei, Int. J. Hydrogen Energy, 2012, 37, 6356–6362 CrossRef CAS.
- C. Italiano, A. Vita, C. Fabiano, M. Laganà and L. Pino, Int. J. Hydrogen Energy, 2015, 40, 11823–11830 CrossRef CAS.
- J. Zhao, W. Zhou and J. Ma, Int. J. Hydrogen Energy, 2014, 39, 16195–16201 CrossRef CAS.
- M. Rezaei, S. M. Alavi, S. Sahebdelfar and Z.-F. Yan, J. Nat. Gas Chem., 2006, 15, 327–334 CrossRef CAS.
- R. Zanganeh, M. Rezaei, A. Zamaniyan and H. R. Bozorgzadeh, J. Ind. Eng. Chem., 2013, 19, 234–239 CrossRef CAS.
- M. Rezaei, S. M. Alavi, S. Sahebdelfar, P. Bai, X. Liu and Z.-F. Yan, Appl. Catal., B, 2008, 77, 346–354 CrossRef CAS.
- X. Huang, N. Sun, G. Xue, C. Wang, H. Zhan, N. Zhao, F. Xiao, W. Wei and Y. Sun, RSC Adv., 2015, 5, 21090–21098 RSC.
- J. Ni, L. Chen, J. Lin, M. K. Schreyer, Z. Wang and S. Kawi, Int. J. Hydrogen Energy, 2013, 38, 13631–13642 CrossRef CAS.
- S. Wang, G. Q. Lu and G. J. Millar, Energy Fuels, 1996, 10, 896–904 CrossRef CAS.
- Z. Alipour, M. Rezaei and F. Meshkani, J. Ind. Eng. Chem., 2014, 20, 2858–2863 CrossRef CAS.
- A. Ranjbar and M. Rezaei, Adv. Powder Technol., 2014, 25, 467–471 CrossRef CAS.
- Z. Mosayebi, M. Rezaei, N. Hadian, F. Z. Kordshuli and F. Meshkani, Mater. Res. Bull., 2012, 47, 2154–2160 CrossRef CAS.
- S. Sepehri, M. Rezaei, G. Garbarino and G. Busca, Int. J. Hydrogen Energy, 2016, 41, 3456–3464 CrossRef CAS.
- B. Li and S. Zhang, Int. J. Hydrogen Energy, 2013, 38, 14250–14260 CrossRef CAS.
- N. Majidian, N. Habibi and M. Rezaei, Korean J. Chem. Eng., 2014, 31, 1162–1167 CrossRef CAS.
- C. Ding, W. Liu, J. Wang, P. Liu, K. Zhang, X. Gao, G. Ding, S. Liu, Y. Han and X. Ma, Fuel, 2015, 162, 148–154 CrossRef CAS.
- S. K. Yadav and P. Jeevanandam, J. Alloys Compd., 2014, 610, 567–574 CrossRef CAS.
- L.-A. Wu, X. Qiao, S. Cui, Z. Hong and X. Fan, Microporous Mesoporous Mater., 2015, 202, 234–240 CrossRef CAS.
- J. Akl, T. Ghaddar, A. Ghanem and H. El-Rassy, J. Mol. Catal. A: Chem., 2009, 312, 18–22 CrossRef CAS.
- M. Tiecco, L. Testaferri, F. Marini, S. Sternativo, F. Del Verme, C. Santi, L. Bagnoli and A. Temperini, Tetrahedron, 2008, 64, 3337–3342 CrossRef CAS.
- A.-L. Brocas, C. Mantzaridis, D. Tunc and S. Carlotti, Prog. Polym. Sci., 2013, 38, 845–873 CrossRef CAS.
- A. I. Tsyganok, T. Tsunoda, S. Hamakawa, K. Suzuki, K. Takehira and T. Hayakawa, J. Catal., 2003, 213, 191–203 CrossRef CAS.
- J. M. Rynkowski, T. Paryjczak and M. Lenik, Appl. Catal., A, 1995, 126, 257–271 CrossRef CAS.
- J. Min, Y. Lee, H. Park, C. Zhang and K. Jun, J. Ind. Eng. Chem., 2015, 26, 375–383 CrossRef CAS.
- Z. Rong-jun, X. Guo-fu, L. Ming-feng, W. Yu, N. Hong and L. Da-dong, J. Fuel Chem. Technol., 2015, 43, 1359–1365 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |