Highly stable polysulfone solvent resistant nanofiltration membranes with internal cross-linking networks
Dongju Chen*a,
Xue Liua,
Dandan Lia and
Xianfeng Li*b
aSchool of Chemistry and Chemical Engineering, Liaoning Normal University, Huanghe Road 850, Dalian 116029, P. R. China. E-mail: dongju.chen@yahoo.com
bDivision of Energy Storage, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, P. R. China. E-mail: lixianfeng@dicp.ac.cn
First published on 4th March 2016
Abstract
Polysulfone based solvent resistant nanofiltration membranes with internal cross-linking networks are designed and fabricated. The internal cross-linking networks are constructed by reacting imidazole with chloromethylated polysulfone (CMPSF). The cross-linking networks can dramatically improve the solvent stability of the polysulfone membranes and the performance of the membrane is tuned via changing the polymer concentration from 20 wt% to 28 wt%. The weight of the cross-linked membranes has barely changed even after immersing them in N,N-dimethylacetamide (DMAc) for more than 96 h, exhibiting superior stability in apolar solvents. The separation performance of the cross-linked PSF membranes is investigated via filtration experiments, where three dyes, crystal violet (CV), bromothymol blue (BTB) and rose bengale (RB) with different MW and different charge properties are selected as solutes. It is found that with increasing polymer concentration, an increased rejection and a decreased flux are observed. The prepared cross-linked membranes showed good selectivity on dyes with different molecular weights in different solvents (yielding more than 99% retention for RB in apolar DMAc) coupled with remarkable stability, exhibiting very good prospects in SRNF application.
Introduction
Separation processes play a dominate role in the chemical and pharmaceutical industries, due to the fact that they account for more than 40% of the capital and operating costs.1 Membrane separation processes gained wide attention due to their advantages of energy saving, environmental friendliness and low cost.2,3 As one kind of membrane process, nanofiltration (NF) allows for the separation and purification of compounds with molecular weight cut-offs (MWCOs) between 200 and 1000 Da over a membrane.4 However, the valuable membranes are in most cases not stable in harsh and corrosive environments. Therefore, to broaden this process to organic feeds, so called solvent resistant nanofiltration (SRNF), is one of the great challenges.5,6SRNF has proved to show great promise in applications like product purification and concentration, solvent exchange and recycling as well as recovery of homogeneous catalysts due to its lower energy consumption and the milder conditions that chemical compounds experience during separation.1,6,7 However, the SRNF process brings a significant challenge for the membranes from a materials point of view, in particular, due to the required solvent-stability, which many traditional polymer membranes lack.8
The most extensively studied polymer for SRNF is based on cross-linked polyimide (PI), which is fabricated via post-cross-linking of asymmetric polyimide nanofiltration membranes.9–11 Even cross-linked polyimide membranes can withstand most solvent systems including strong polar solvents, but their stability in acidic and basic media needs to be further improved. Apart from stability, tailored membrane performance is a desirable feature of PI membranes as well. Other traditional aromatic polymers like poly (ether ether ketone) and polysulfone show very stable performance in strong acidic and basic media,12,13 however, they can only be used in certain solvents, e.g. alcohols, and can never withstand strong polar solvents, which is one of the most troublesome issues in SRNF. Therefore to improve the solvent stability of these polymer membranes is very critical and very beneficial for broadening SRNF application.
Cross-linking has been proved to be a very powerful way to improve the stability of polymer membranes and to form highly stable SRNF membranes. Apart from the most studied polyimide, cross-linking reactions are widely used in SRNF membrane fabrication. Polysulfone, as one kind of aromatic polymer, is widely applied in nanofiltration processes due to its outstanding mechanical and thermal stability.14 For SRNF applications, polysulfone based membranes can only withstand alcohols. Even after UV-curing, their performance in polar solvents like DMAc and DMF has been rarely reported.13
Herein, we will present a versatile method to fabricate polysulfone based SRNF membranes with high solvent stability. The method is based on establishing internal cross-linking networks between the polysulfone main chains. The cross-linking networks can dramatically improve the solvent stability of polysulfone membranes. Their separation performance under strong polar solvents will be illustrated for the first time. Apart from the excellent stability, positively charged groups derived from the cross-linking reagents could offer enhanced antifouling properties as well.
Experimental
Preparation of CMPSF
Chloromethyl polysulfone (CMPSF) was prepared by the method as reported:15 15 g of polysulfone (Udel P3500, Solvay) was dissolved in 600 mL of trichloromethane (Tian Jiang Kermel Chemical reagent Co. Ltd), in a three-necked round-bottomed flask at room temperature. Under nitrogen protection, 1200 μL of anhydrous SnCl4 (Sinapharm Chemical reagent Co. Ltd) and 25 mL of chloromethyl methyl ether (Nan Jing Hengchang Biomedical Co. Ltd.) were added slowly. The hybrid solution was stirred at 55 °C for 24 h. The mixture was precipitated and washed using methanol and dried under vacuum at 50 °C for 24 h.CMPSF membrane preparation
The CMPSF nanofiltration membrane was prepared using a typical phase inversion method. CMPSF was first dissolved in dimethylacetamide (DMAc) to form a solution. The solution was then cast on a clean and dust-free glass plate, and the glass plate was transferred to a water bath. Afterward, the membranes were peeled off and soaked in deionized water before cross-linking.Membrane cross-linking
Membranes with a fixed size of 11.4 × 9.0 cm2 were immersed in 300 mL of a 10 wt% imidazole solution in water for 30 hours. Afterward, the membranes were washed with and stored in deionized water for use.Characterization
The chemical structure of the membranes was characterized using a JASCO FTIR 4100 spectrometer. Each spectrum was recorded at an average rate of 48 scans with a resolution of 4 cm−1, collected from 600 to 4000 cm−1 in absorption mode. SEM (JEOL JCM-6000) was applied to observe the cross-sectional morphology of the membranes. The cross-section was obtained by breaking the membranes in liquid nitrogen. The samples were coated with gold before SEM analysis. The surface morphology of the membranes was characterized using field emission scanning electron microscopy (FE-SEM, SUPRA 55).Membrane stability
A dried membrane with a size of 6 cm × 6 cm was first weighed and then immersed in 50 mL of DMAc solution at room temperature. At different time intervals, the membrane was taken out, dried and weighed.Filtration experiment
All filtration experiments were performed at room temperature on a stainless steel dead-end pressure cell with a membrane area of 19.0 cm2. A feed solution of 35 μM solute in a certain solvent was added into the cell and the pressure was kept at 20 bar. The solute properties used in the filtration experiments are listed in Table 2. The permeance J was calculated by using eqn (1), where V is the total volume of permeated solvent, A is the effective filtration area, Δt is the flow time across the membrane, and ΔP is the operating pressure.
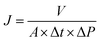 | (1) |
The concentration of the solutes in the permeate (Cp) and the initial feed solution (Cf) was measured using a TU-1901 double-beam UV-vis spectrophotometer. The effective solute rejection was calculated using eqn (2):
 | (2) |
Results and discussion
To realize the idea, the polysulfone was firstly chloromethylated via a typical nucleophilic substitution reaction to form chloromethylated polysulfone (CMPSF).16 The degree of chloromethylation obtained by 1H NMR was about 1.28 (Fig. 1).17 Then nanofiltration membranes based on CMPSF were prepared via a typical phase inversion method.18 The morphology of the CMPSF was tuned via changing the polymer concentration, where polymer concentrations of 20 wt%, 22 wt%, 25 wt% and 28 wt% were selected. The resulting membranes are referred to as CMPSF-20, CMPSF-22, CMPSF-25 and CMPSF-28 respectively. Afterwards, the CMPSF was further cross-linked by imidazole as shown in Scheme 1.The cross sectional morphologies of the pristine prepared CMPSF nanofiltration membranes are shown in Fig. 2a–d. The corresponding membranes are referred to as M-CMPSF-X. All the membranes show a typical asymmetric macrovoid-like structure, which includes a porous support (Fig. 2a–d) and a thin skin layer (Fig. 2a–d). With increasing concentration of CMPSF from 20% to 28%, fewer macrovoids were found and the cross section becomes more spongy. The solution was too viscous when the concentration exceeded 28%, where a uniform membrane is difficult to make. The morphology changes can be explained by the kinetic aspect relating to the increase in polymer concentration. The viscosity of the solution will increase with increasing concentration, further lowering the exchange rate between the solvent and non-solvent.19 A denser skin layer will be formed and further suppress the formation of macrovoids and induce the formation of sponge like pores.
Afterward, the membranes were treated with 10 wt% imidazole solutions for 30 hours for further cross-linking. The optimized cross-linking time was determined via changing the cross-linking time from 10 to 36 hours, where their separation performance was determined by filtrating RB in acetone and DMAc (Fig. 3). The flux first decreases and then reaches a constant (Fig. 3b) and the rejection first increases and then reaches a constant (Fig. 3a) with increasing cross-linking time, where an optimized condition of 30 hours was selected for the following experiments to ensure the complete cross-linking reaction.
 | ||
| Fig. 3 The separation performance of CMPSF-25 with different cross-linking times. (a) Rejection performance; (b) permeance performance. | ||
The morphology of the membranes after cross-linking in Fig. 2e–h indicated overall similar structures with the non-cross linked ones. However, the skin layer (Fig. 2e–h) becomes denser after cross-linking due to the formed internal cross-linking networks, which will be expected to show higher selectivity.
Fig. 4 shows a typical FTIR spectrum before and after cross linking. The absorption band at around 740 cm−1 in the spectrum of the CMPSF membrane is attributed to the characteristic absorption of the C–Cl in CMPSF, confirming the successful introduction of chloromethyl groups in PSF. Compared with the pristine CMPSF, a new absorption at around 1504 cm−1, attributed to the stretching of the C–N bond in imidazole, was clearly observed in the cross linked membranes, indicating the successful introduction of imidazole groups in the prepared membranes. Meanwhile, the band at 740 cm−1 disappeared after introducing imidazole into the CMPSF, suggesting that the chloromethyl groups reacted almost completely with imidazole.
The separation properties of the CMPSF nanofiltration membranes were investigated via filtration experiments on different dyes in IPA and ethanol solutions. As shown in Table 1, all the membranes show very low or even no retention on all dyes. With increasing polymer concentration, the permeability shows an obvious decreasing tendency, which can be explained by the membrane morphology.
| Membrane | Et-RB | IPA-RB | Et-CV | IPA-CV | Et-BTB | IPA-BTB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ra | Pb | R | P | R | P | R | P | R | P | R | P | |
| a R: rejection (%).b P: permeability (L m−2 h−1 bar−1). | ||||||||||||
| CMPSF-20 | 1.79 | 6.27 | 0.03 | 1.62 | 0.10 | 3.51 | 2.10 | 1.34 | 0.03 | 4.84 | 0.04 | 1.27 |
| CMPSF-22 | 2.41 | 5.80 | 0.65 | 1.64 | 0 | 3.38 | 4.21 | 1.15 | 0.86 | 3.81 | 0.61 | 1.30 |
| CMPSF-25 | 2.70 | 3.37 | 1.24 | 1.14 | 16.6 | 2.16 | 11.0 | 1.07 | 4.58 | 2.22 | 7.27 | 0.97 |
| CMPSF-28 | 3.23 | 1.97 | 1.91 | 0.80 | 27.1 | 1.37 | 12.0 | 0.96 | 4.87 | 1.52 | 6.75 | 0.53 |
The solubility of membranes in different solvents before and after cross-linking is indicated in Table 2. After cross-linking, the solvent stability of polysulfone based membranes improved dramatically. For example, before cross-linking, the membrane is only resistant to alcohol based solvents. After modification, the membranes are resistant to all the solvents selected, especially the apolar solvents like DMF and DMAc. The results indicated that the cross-linked polysulfone membranes showed remarkable stability in different solvents.
| Ethanol | IPA | Acetone | DMF | |
|---|---|---|---|---|
| a (−): not dissolvable, (+): dissolvable. | ||||
| CMPSF-25 | − | − | + | + |
| M-CMPSF-25 | − | − | − | − |
To further confirm the stability of the membranes in different solvents, the cross-linked membranes were immersed in DMAc and acetone for different times. The weight of the membranes barely changed even after more than 96 hours, which further confirmed the excellent stability of the cross-linked membranes (Fig. 5a).
The separation performance of the cross-linked PSF membranes was investigated via filtration experiments. Dyes with different MW and different charge properties (Table 3) were selected as solutes. Among the dyes, RB carries a negative charge, while CV possesses a positive charge and BTB is neutral. The MW of the selected dyes is in the order of RB > BTB > CV. Acetone and DMAc were selected as solvents (Fig. 5b and c). As expected, all the membranes showed increasing retention for the different dyes and decreasing permeability with increasing polymer concentration. For example, the rejection of cross-linked PSF membranes for RB in acetone increases from 84% to 99% (Fig. 5b), when increasing polymer concentration from 20% to 28%. Meanwhile, their permeance decreases from 1.32 to 0.14 L m−2 h−1 bar−1 (Fig. 5b). Compared with pristine PSF membranes, the cross-linked membranes showed dramatically improved retention of different dyes, due to the fact that the pristine membranes even show no retention for any of the above solutes. Note that the prepared membranes show very promising separation properties and stability in strong polar solvents. All the cross-linked PSF membranes showed more than 95% retention for RB in DMAc solutions (Fig. 5c). When employing acetone as the solvent, the retention for the dyes follows the order of RB > BTB > CV, which agrees well with the molecular weight tendency, indicating that size exclusion will be the dominate transport mechanism of these dye solutions in acetone. Meanwhile, the tendency becomes less obvious when using DMAc as the solvent, which is possibly due to the different interactions between the solvent and solute or the solvent and the membranes.19 To further investigate the stability of the prepared membranes, an M-CMPSF-25 membrane was selected for an extended filtration experiment in DMAc to prove the long-term stability of the modification under these demanding conditions. The membrane showed a clearly stable permeability and retention over 28 hours (Fig. 5d), further confirming the very high stability of the cross-linked membranes. Compared with other reported polysulfone based SRNF membranes, the prepared membranes show better stability in different solvents especially in a strong apolar solvent like DMAc, where the traditional polysulfone based membranes are not resistant.12
Table 4 shows the rejection of cross-linked membranes for RB using different solvents. All membranes show rejection in the order of DMAc > acetone > ethanol > IPA, while, the permeance is in the order of DMAc–IPA > ethanol > acetone, which follows the solvent viscosity very well. The high rejection for DMAc based solutes may be due to the interaction between imidazole groups from the membranes and amine groups from the DMAc. The similar polarity of these groups could possibly induce high rejection. On the other hand, by using IPA, the strong interaction between OH and imidazole could possibly be beneficial for the transportation of solutes, further lowering the rejection. Overall, the prepared cross-linked membranes show very impressive separation performance for SRNF application.
| Membrane | Ethanol | IPA | Acetone | DMAc | ||||
|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | |
| M-CMPSF-20 | 58.6 | 1.82 | 57.8 | 0.61 | 84.2 | 1.32 | 98.4 | 0.19 |
| M-CMPSF-22 | 74.2 | 0.83 | 72.2 | 0.69 | 98.9 | 0.18 | 99.1 | 0.16 |
| M-CMPSF-25 | 90.8 | 0.65 | 87.3 | 0.05 | 97.1 | 0.10 | 99.1 | 0.05 |
| M-CMPSF-28 | 95.4 | 0.29 | 82.0 | 0.09 | 99.3 | 0.14 | 96.9 | 0.05 |
Conclusions
In summary, a kind of polysulfone membranes with internal cross-linking networks was designed and first used in a solvent resistant nanofiltration application. The membranes were prepared via cross-linking CMPSF with imidazole. The morphology of the membranes was tuned via changing the polymer concentration from 20 wt% to 28 wt%. With increasing the concentration from 20 wt% to 28 wt%, the pore size decreased, where an increased rejection and a decreased flux were found. The prepared membranes showed remarkable stability after cross-linking. The membranes show very good selectivity on dyes with different MW in different solvents. Especially, the prepared membranes show more than 99% retention for RB in apolar DMAc, showing very good prospects in SRNF application.Acknowledgements
The authors greatly acknowledge the financial support from the China Natural Science Foundation (Grant No. 21476224) and the Outstanding Young Scientist Foundation, Chinese Academy of Sciences (CAS) and the Dalian Municipal Outstanding Young Talent Foundation (2014J11JH131).Notes and references
- P. Marchetti, M. F. Jimenez Solomon, G. Szekely and A. G. Livingston, Chem. Soc. Rev., 2014, 114, 10735–10806 CrossRef CAS PubMed.
- R. Rautenbach and R. Albrecht, Membrane Processes, John Wiley & Sons, 1989 Search PubMed.
- H. Strathmann, J. Membr. Sci., 1981, 9, 121–189 CrossRef CAS.
- A. Mohammad, Y. Teow, W. Ang, Y. Chung, D. Oatley-Radcliffe and N. Hilal, Desalination, 2015, 356, 226–254 CrossRef CAS.
- X. Q. Cheng, Y. L. Zhang, Z. X. Wang, Z. H. Guo, Y. P. Bai and L. Shao, Adv. Polym. Technol., 2014, 33 DOI:10.1002/adv.21455.
- P. Vandezande, L. E. Gevers and I. F. Vankelecom, Chem. Soc. Rev., 2008, 37, 365–405 RSC.
- A. Volkov, D. Stamatialis, V. Khotimsky, V. Volkov, M. Wessling and N. Plate, Desalination, 2006, 199, 251–252 CrossRef.
- H. Siddique, E. Rundquist, Y. Bhole, L. Peeva and A. Livingston, J. Membr. Sci., 2014, 452, 354–366 CrossRef CAS.
- J. da Silva Burgal, L. G. Peeva, S. Kumbharkar and A. Livingston, J. Membr. Sci., 2015, 479, 105–116 CrossRef CAS.
- K. Vanherck, P. Vandezande, S. O. Aldea and I. F. Vankelecom, J. Membr. Sci., 2008, 320, 468–476 CrossRef CAS.
- K. Vanherck, G. Koeckelberghs and I. F. Vankelecom, Prog. Polym. Sci., 2013, 38, 874–896 CrossRef CAS.
- I. Strużyńska-Piron, J. Loccufier, L. Vanmaele and I. F. Vankelecom, Chem. Commun., 2013, 49, 11494–11496 RSC.
- J. Yin, G. Zhu and B. Deng, J. Membr. Sci., 2013, 437, 237–248 CrossRef CAS.
- J. Pan, S. Lu, Y. Li, A. Huang, L. Zhuang and J. Lu, Adv. Funct. Mater., 2010, 20, 312–319 CrossRef CAS.
- W. Xu, Y. Zhao, Z. Yuan, X. Li, H. Zhang and I. F. Vankelecom, Adv. Funct. Mater., 2015, 25, 2583–2589 CrossRef CAS.
- P. Van de Witte, P. Dijkstra, J. Van den Berg and J. Feijen, J. Membr. Sci., 1996, 117, 1–31 CrossRef CAS.
- F. Zhang, H. Zhang and C. Qu, J. Mater. Chem., 2011, 21, 12744–12752 RSC.
- E. A. Weiber and P. Jannasch, J. Membr. Sci., 2015, 481, 164–171 CrossRef CAS.
- A. K. Hołda and I. F. Vankelecom, J. Appl. Polym. Sci., 2015, 132, 42130 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |