β-Cyclodextrin included coumarin derivatives as selective fluorescent sensors for Cu2+ ions in HeLa cells†
Abstract
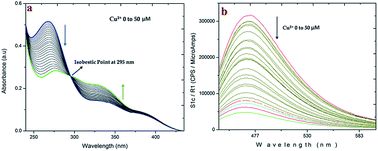
A highly sensitive and selective fluorescent sensor for Cu2+ ions in water medium is reported using 4-((benzo[d]thiazol-2-ylthio)methyl)-5,7-dihydroxy-2H-chromen-2-one (1) included β-cyclodextrin, as a probe. The fluorogenic supramolecule has displayed good selectivity and affinity towards Cu2+ ions over other cations after examining in biological systems with intracellular Cu2+ ions, especially in cultured HeLa cells using fluorescence microscopic imaging. The lowest detection limit of Cu2+ ions observed using this probe is as low as 2.52 × 10−10 M. The observed on-off fluorescence with the periodic addition of Cu2+ ion is explained via an Intramolecular Charge Transfer mechanism (ICT) and the inclusion of 1 in β-cyclodextrin is characterised by 1H-NMR molecular modeling studies. The results show that the present β-CD:1 system, studied in HeLa cells, can be potentially used in monitoring the biological functions of Cu2+ ions.

 Please wait while we load your content...
Please wait while we load your content...