Curcumin induces cell death of human papillary thyroid carcinoma BCPAP cells through endoplasmic reticulum stress
Lixi Zhang†
ab,
Li Zhang†ac,
Xian Chenga,
Yanyan Gaoa,
Jiandong Baoa,
Huixin Yua,
Haixia Guand,
Yang Sunc and
Rongrong Lu*b
aKey Laboratory of Nuclear Medicine, Ministry of Health, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, China
bState Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi, Jiangsu 214122, China. E-mail: lurr@jiangnan.edu.cn
cState Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing, Jiangsu, China
dDepartment of Endocrinology & Metabolism and Institute of Endocrinology, The First Hospital of China Medical University, Shenyang, Liaoning, China
First published on 26th May 2016
Abstract
Curcumin, a major active component of Curcuma longa, is a natural polyphenolic antioxidant compound which has a strong potential for cancer prevention and treatment. However, the effect of curcumin on papillary thyroid carcinoma cells has not been investigated. In the present study, we report that curcumin induces cell death of papillary thyroid carcinoma (PTC) cell line BCPAP cells through endoplasmic reticulum (ER) stress. Curcumin suppressed cell viability of BCPAP cells in a dose-dependent manner. A large body of evidence demonstrates that ER stress contributes to cell death in certain contexts. ER stress was induced by curcumin treatment in BCPAP cells, as evidenced by the up-regulation of intracellular calcium level, and up-regulated expression of CCAAT/enhancer binding protein homologous protein (CHOP) at both mRNA and protein levels compared to the control group. Moreover, the activation of activating transcription factor 6 (ATF6)/X-box binding protein-1 (XBP-1) pathways could be involved in the ER stress signaling of curcumin treatment. Thus, our results provide new insights into the molecular mechanisms of curcumin-mediated cell death in PTC cells and suggest curcumin might be a potential chemotherapeutic agent which is able to fight against papillary thyroid cancer.
1. Introduction
Thyroid cancer is the most common malignant endocrine tumor. The well-differentiated papillary carcinoma (PTC) is one of the major thyroid tumors.1 In the past 30 years, there has been a steady increase in all thyroid cancers, especially in PTC with a nearly tripled incidence in the general population.2 Conventional therapies for PTC include surgical resection, radioactive iodine and thyroid hormone suppression therapy.3 The prognosis of PTC is usually good, and the survival rate of 10 years is about 85%. However, the recurrence of PTC is rising to about 33% and only 30% of patients have complete remission after radioiodine therapy. Therefore, new therapeutic strategies need to be developed in thyroid cancer treatments.Nowadays, natural products play a more and more significant role in drug discovery. Curcumin is a natural phytochemical derived from the popular Indian spice turmeric plant.4 Curcumin displays broad pharmacological activities which can be used to treat various diseases, including arthritis, digestive and liver abnormalities, respiratory infections and cancer.5,6 Recent studies have demonstrated that curcumin could inhibit esophageal cancer growth,7 prostate cancer progression to metastasis,8 cervical cancer8 and so on. Previously, our group demonstrated that curcumin could induce apoptosis in K1 papillary thyroid cancer cells and it also inhibits the basal9,10 and hypoxia-induced migration ability of K1 cells.11 Considering the various molecular targets of curcumin, the precise molecular mechanisms by which curcumin inhibits the growth of human thyroid cancer cells are complex, not fully elucidated.
The endoplasmic reticulum (ER) is an intracellular organelle which is responsible for protein folding, protein modification and protein transportation.12 Alterations in ER homeostasis lead to the accumulation of unfolded or misfolded proteins in the ER lumen, which is called ER stress.12–14 Once the ER stress happened, these sensors will be activated.15 If the ER stress prolonged, it can lead to cell death through apoptosis.16 Therefore, ER stress plays an important role in cancer development, such as human hepatocellular carcinoma,13 human melanoma cancer17 and human cervical cancer18 and thyroid cancer.19,20 ER stress regulate the sensitivity of thyroid cancer cells to apoptosis via regulating the ER stress-related molecules, including CCAAT/enhancer binding protein homologous protein (CHOP), activating transcription factor 6 (ATF6), X-box binding protein-1 (XBP-1) an so on.19 Previous studies have shown that curcumin could induce cell death through ER stress in human CD4+ T cells,21 in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells,22 human non-small cell lung cancer NCI-H460 cells23 and so on. However, little is known about the mechanism of curcumin-induced ER stress in the thyroid cancer.
In general, curcumin can impact ER stress, and thereby could be used as a promising therapy for diseases.21 Thus, our work demonstrated the molecular pathway during the curcumin-induced ER stress in the BCPAP cells via regulating the level of calcium, CHOP and the XBP-1 splicing.
2. Experimental
2.1. Chemicals, reagents and antibodies
Curcumin (Cur, catalog No. C7727) was purchased from Sigma Aldrich. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sangon (Shanghai, China). Rhod-2/AM (Dojindo, USA), anti-CHOP, anti-ATF6 and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology. All other chemicals were of analytical reagent grade and purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).2.2. Cell culture and drug treatment
Papillary thyroid cancer cell line BCPAP was obtained from the German Collection of Micro-organisms and Cell Cultures (Braunschweig, Germany) and maintained in RPMI 1640 containing 10% new born bovine serum, 100 U mL−1 penicillin and 100 U mL−1 streptomycin in a humid atmosphere of 5% (v/v) CO2 and 95% (v/v) air at 37 °C. BCPAP cells in the log phase were plated in cell culture plates (Corning, NY, USA). Curcumin was dissolved in DMSO at 50 mM as a stock solution and stored at −20 °C until diluted before use. The solvent control contained an equivalent amount of DMSO corresponding to the highest used concentration of curcumin.2.3. Assessment of cell viability
Cell viability was analyzed using the MTT assay, which measures the ability of metabolic active cells to form formazan through cleavage of the tetrazolium ring of MTT.24 In brief, cells were treated with curcumin in quadruplicate in a 96-well plate for 24 h. Then, 40 μL of MTT solution (2 mg mL−1) was added to each well and incubated for another 4 h at 37 °C. The supernatants were aspirated carefully and 100 μL of DMSO was added, and then the plate was holding on vibrator for 5 min. The optical density of the cell suspension was measured at 490 nm using a microplate reader (Bio-Tek instruments Inc., Winooski, VT). Cell viability was expressed as a percentage of MTT reduction, assuming that the absorbance of untreated cells was 100%.2.4. Reverse transcriptase-polymerase chain reaction (RT-PCR)
After cell collection, the total RNA was extracted from the cells using the TRIzol reagent (Ambion, USA), as described by the manufacturer. First-strand cDNAs were generated by reverse transcription using oligo (dT) from RNA samples. The primer sequences (Sangon, Shanghai, China) were as follows: CHOP, forward: 5′-ACCAGGAAACGGAAACAG-3′, reverse: 5′-TGCGTATGTGGGATTGAG-3′; GRP78, forward: 5′-TCAGGGCAACCGCATCAC-3′, reverse: 5′-CGCCACCCAGGTCAAACA-3′; XBP-1, forward: CCTTGTAGTTGAGAACCAGG, reverse: GGGGCTTGGTATATATGTGG; Serp1, forward: ATGGTCGCCAAGCAAAGG, reverse: TCACATGCCCATCCTGAT and β-actin, forward: 5′-GCCGGGACCTGACTGACTAC-3′, reverse: 5′-CGGATGTCCACGTCACACTT-3′. The PCR was performed using an initial step of denaturation at 95 °C for 5 min, with 30 cycles of amplification at 95 °C for 30 s, annealing at different temperature from 55 to 65 °C respectively for 30 s, elongation at 72 °C for 30 s, and extension at 72 °C for 5 min. The PCR products were electrophoresed in 1.5% agarose gel and visualized by ethidium bromide (EB) dying. The relative expression was quantified densitometrically using the GIS-2019 system (Tanon, Shanghai, China), and calculated according to the reference bands of β-actin.2.5. Intracellular calcium assay
Intracellular Ca2+ levels were determined with the Ca2+-sensitive fluorochrome Rhod-2/AM which can cross the cell membrane and be cut into Rhod-2 by intracellular esterase.25 The Rhod-2 can specifically combine with the Ca2+ and emit a strong fluorescence. Cells treated with 12.5 to 50 μM curcumin for 24 h were collected and washed twice with D-hanks. After that, cells were incubated with 5 μM of Rhod-2/AM at 37 °C for 45 min in the dark and gently washed twice with D-hanks. The fluorescence was analyzed by flow cytometry (FACSAria, Bectoa Dickinson, USA) through FL2 channel.2.6. Western blot assay
BCPAP cells treated with different concentrations of curcumin (12.5, 25 and 50 μM) for 24 h were collected, and then the Western blot analysis was carried out as previously described with some modifications.25 After centrifugation, cells were lysed in 20 μL of lysis buffer [150 μM NaCl, 1% (w/v) NP-40, 0.02% (w/v) NaN3, 10 μg mL−1 PMSF, 50 μM Tris–HCl (pH 8.0)] containing supplementary protease inhibitor. The lysate was subjected to repeated freezing and thawing for three times, and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C. The supernatant was collected and the protein concentration was determined using Bradford assay. After addition of sample loading buffer, protein samples were analyzed with electrophoresis on 10% SDS-PAGE and subsequently transferred onto a nitrocellulose membrane (Millipore, USA). The membrane was incubated in fresh blocking buffer [0.1% (v/v) Tween 20 in Tris-buffered saline, pH 7.4, containing 5% (w/v) skim milk] at room temperature for 1 h and then probed with the following antibodies: anti-β-actin (1
000 rpm for 15 min at 4 °C. The supernatant was collected and the protein concentration was determined using Bradford assay. After addition of sample loading buffer, protein samples were analyzed with electrophoresis on 10% SDS-PAGE and subsequently transferred onto a nitrocellulose membrane (Millipore, USA). The membrane was incubated in fresh blocking buffer [0.1% (v/v) Tween 20 in Tris-buffered saline, pH 7.4, containing 5% (w/v) skim milk] at room temperature for 1 h and then probed with the following antibodies: anti-β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, v/v), anti-CHOP (1
1000, v/v), anti-CHOP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v/v), anti-GRP78 (1
100, v/v), anti-GRP78 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, v/v) in blocking buffer at 4 °C overnight. After three times washing each for 5 min with TBST (Tris-buffered saline with 0.1% (v/v) Tween 20), the membrane was incubated with the appropriate HRP-conjugated secondary antibody at room temperature for 1 h and then washed again three times in TBST buffer. The membrane was incubated with enhanced chemiluminescence substrate solution (cell signaling) for 5 min according to the manufacturer's instructions and visualized with autoradiography film.
1000, v/v) in blocking buffer at 4 °C overnight. After three times washing each for 5 min with TBST (Tris-buffered saline with 0.1% (v/v) Tween 20), the membrane was incubated with the appropriate HRP-conjugated secondary antibody at room temperature for 1 h and then washed again three times in TBST buffer. The membrane was incubated with enhanced chemiluminescence substrate solution (cell signaling) for 5 min according to the manufacturer's instructions and visualized with autoradiography film.
2.7. Detection of XBP-1 mRNA splicing
XBP-1 mRNA splicing was analyzed using RT-PCR methods with XBP-1 specific primers. Cycling conditions were 95 °C for 5 min, followed by 30 cycles of 95 °C for 30 s, 57 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min. We separated the PCR products by electrophoresis on a 3.5% agarose gel and visualized them by ethidium bromide staining.26 The unspliced XBP1 mRNA has a PstI site within the 26 nt intron, so the DNA fragment was digested with PstI at 37 °C overnight, which could be cleaved to 2U and 3U. Subsequent electrophoresis revealed the inactive form as two cleaved fragments and the active form as a noncleaved fragment.2.8. Statistical analysis
Results were expressed as mean ± SEM or mean ± SD as indicated. Two group comparisons were evaluated using the Student's t-test. Differences were considered statistically significant when P < 0.05.3. Results
3.1. Curcumin inhibits the cell viability of BCPAP cells
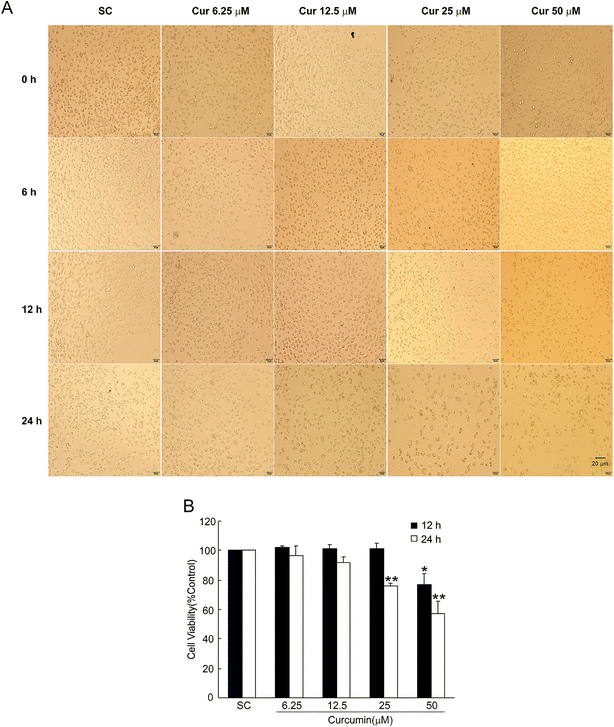
We used the MTT assay to determine the viability of BCPAP cells treated with different concentrations (6.25, 12.5, 25 and 50 μM) of curcumin for 0, 6, 12 and 24 h, respectively. As shown in Fig. 1A, the number of cells decreased as the curcumin concentration increased. Compared with the solvent control, the cell morphology was not changed with low concentrations (6.25, 12.5 μM) of curcumin for 12 and 24 h. But under the higher concentrations (25, 50 μM) of curcumin treatment for 24 h, the cell number was decreased and the BCPAP cells shrunk and clustered. Moreover, curcumin decreased the viability rate of BCPAP cells in dose-dependent and time-dependent manners. As shown in Fig. 1B, in comparison with the control groups, curcumin treatment at 25 and 50 μM for 24 h significantly (P < 0.01) decreased the viability rate to 75.60 ± 1.93% and 56.72 ± 9.14%, respectively. These results indicated that curcumin could inhibit the cell viability of BCPAP cells.3.2. Curcumin increases the level of intracellular Ca2+
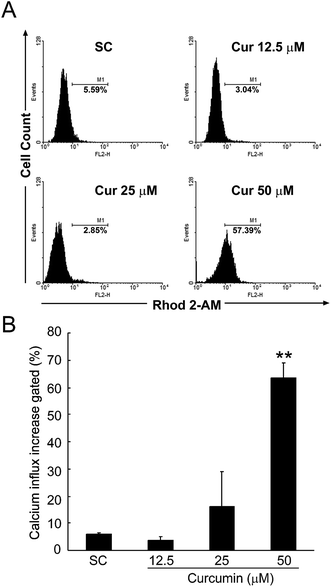
The ER is the major intracellular Ca2+ store, where it regulates several processes such as protein folding. The dysregulation of the homeostasis of ER Ca2+ can activate cell death pathways.24 Therefore the level of intracellular Ca2+ is an important marker of ER stress. As shown in Fig. 2, after 24 h treatment with 50 μM of curcumin, the level of intracellular Ca2+ significantly increased in the BCPAP cells (P < 0.01). Taken together, these data suggested that curcumin triggered ER stress via regulation of the intracellular Ca2+ level.3.3. Curcumin regulates the mRNA and protein expression levels of ER stress marker
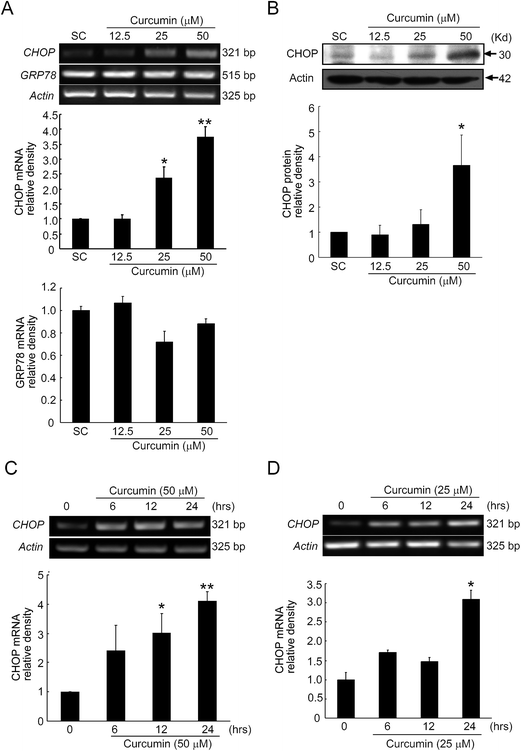
CHOP is an apoptotic transcriptional factor which is close related with ER stress. GRP78 is a prominent ER-resident chaperone, which binds to three ER stress sensors including ATF6, PERK, IRE1α, but more stably interacts with misfolded or unfolded proteins.15 Therefore, CHOP and GRP78 are commonly used as ER stress markers. To evaluate whether curcumin could induce ER stress, we determined the levels of CHOP and GRP78 in BCPAP cells treated with different concentrations of curcumin. As shown in Fig. 3A and C, curcumin dose-dependently and time-dependently up-regulated the mRNA expression level of CHOP. In addition, we found that curcumin with low concentration also time-dependently up-regulated the mRNA expression level of CHOP, which indicated that ER stress could also be activated with low concentration of curcumin treatment (Fig. 3D). In contrast to CHOP, the mRNA level of GRP78 was unaffected by curcumin treatment. Further western blot analyses revealed that curcumin up-regulated the protein level of CHOP as expected (Fig. 3B). These results showed that curcumin could up-regulate the level of CHOP, indicating that curcumin induced ER stress in the BCPAP cells.3.4. Curcumin induces ER stress through ATF6/XBP-1 signaling pathway
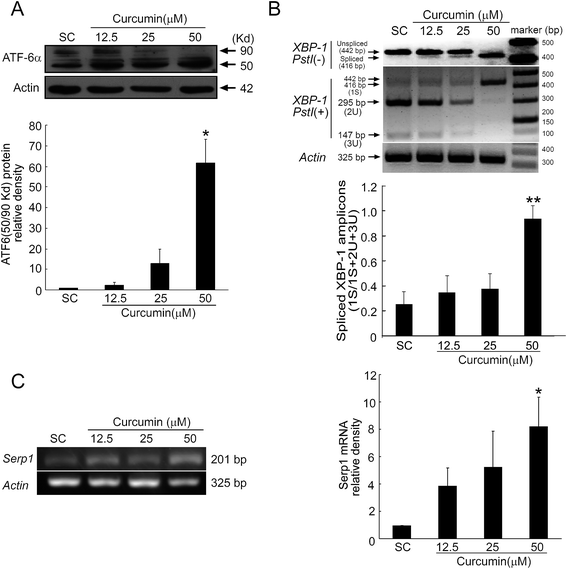
Induction of ER stress leads to activation of UPR to restore ER homeostasis.27 The unfolded protein response (UPR) is initiated through three transmembrane proteins, including inositol-requiring enzyme 1 (IRE1), pancreatic ER kinase (PERK) and activating transcription factor 6 (ATF6).28 The IRE1α and ATF6 pathways of the UPR converge on XBP-1. In response to ER stress, ATF6 is cleaved and activated. Once ATF6 was cleaved, the cleaved ATF6 stimulated the X-box binding protein-1 (XBP-1u) splicing via cleaving a 26-base intron from the XBP-1 mRNA, leading to the up-regulated expression of activated XBP-1 (XBP-1s).29 We then test whether the cleavage expression of ATF6 was up-regulated with different concentrations of curcumin and whether the activated ATF6 enhanced the XBP-1 splicing. As shown in Fig. 4A, curcumin induced the increased expression of ATF6 cleavage (50 kDa) accompanying with a decline of ATF6 (90 kDa). Moreover, we found that treatment with curcumin ranging from 12.5 to 50 μM for 24 h decreased the mRNA expression of XBP-1u and activated the splicing XBP-1 (XBP-1s), comparing with the solvent controls. These results indicated that ER stress induced by curcumin could activate the ATF6 and cause the XBP-1 splicing.SERP1, which is a downstream target gene of XBP-1, controls biogenesis of secretory proteins and stabilizes newly synthesized membrane proteins during ER stress.30,31 Next, we detected the expression of SERP1. As shown in Fig. 4C, the mRNA expression of SERP1 was up-regulated after curcumin treatment in the BCPAP cells. Taken together, these results showed that curcumin could induce the ATF6 cleavage and enhance the XBP-1 splicing during ER stress.
4. Discussion
Conventional treatments, such as chemotherapy and radiotherapy, which induce apoptosis, are often initially effective in different kinds of cancers. For many patients with differentiated thyroid cancer (DTC), radioiodine ablation of a thyroid remnant after near-total thyroidectomy, which belongs to radiotherapy, is a well-accepted therapy to manage locoregional and metastatic spread.32,33 Most DTC patients have an excellent outlook with the use of traditional therapies, including radioactive iodine (RAI) ablation of the remaining thyroid remnant. However, about 25–50% of locally advanced or metastatic patients become refractory to radioactive iodine (RAI). When RAI becomes ineffective against DTC, the long-term survival will be affected. At this point, there is a clear unmet need to increase the sensitivity to RAI.34 It is known that apoptosis is an essential process of eliminating destined cells after radiotherapy.35 And previous studies show that radiation resistance is associated with apoptosis resistance.36 Then we surmise that enhancing the extent of apoptosis would reduce the resistance to the radioactive iodine. Unfortunately, current approaches to increase the sensitivity to RAI of the thyroid cancer are limited.37Curcumin, a kind of natural product, shows a vital role in cancer prevention and treatment through modulation of various biological activities,5 including cell cycle, apoptotic signals, miRNAs, Wnt/beta-catenin signaling, nuclear factor-κB and so on. Recently, some studies reported that curcumin could induce apoptosis via ER stress in the human CD4+ T cells,21 human gastric carcinoma cells, colon carcinoma22 and so on. Under moderate ER stress, the UPR pathways would be activated to maintain the homeostasis. When the cellular damage exceeds the capacity of the adaptive response, ER stress is prolonged and continued activation of the Unfolded Protein Response (UPR) signals the cell for apoptosis.16 Thus, the signaling pathways of ER stress will hopefully be a new possibility for cancer treatment. Currently, curcumin has been applied for several clinical trials including pancreatic cancer, colorectal cancer, multiple myeloma and Alzheimer's.38 Previous studies in our laboratory indicated that curcumin could induce apoptosis in K1 papillary thyroid cancer cells.9,10 However, the detailed mechanism remains unclear. In the present study, we investigated whether curcumin could induce apoptosis via triggering the ER stress in the BCPAP cells.
We first examined the viability of BCPAP cells with different concentrations of curcumin for indicated periods. We found that curcumin (25 and 50 μM) could apparently inhibit the cell viability after 24 h treatment (Fig. 1). As the endoplasmic reticulum (ER) is an intracellular Ca2+ store that participates in the regulation of Ca2+ homeostasis. When the ER function is disturbed, ER stress would happen. Moreover, ER stress breaks the calcium homeostasis, which causes a release of Ca2+ from the ER and an increase in cytosolic free Ca2+ level.12,39 Consistent with this notion, curcumin increased the level of intracellular calcium in BCPAP cells (Fig. 2), indicating that curcumin broke the Ca2+ homeostasis and caused the release of Ca2+ from the ER.
Furthermore, ER stress activates the UPR, which includes three signaling pathways. The UPR of cells is initiated by three ER transmembrane proteins, IRE1α (inositol-requiring protein-1α), PERK (protein kinase RNA (PKR)-like ER kinase), and ATF6 (activating transcription factor 6).17 The function of UPR signaling is usually to promote cell survival by balancing the protein load and the folding in the ER. When the cells are exposed to the prolonged ER stress, the cells will die owing to apoptosis. CCAAT/enhancer-binding protein-homologous protein (CHOP) is a major pro-apoptotic transcription factor which mediates ER-stress induced apoptosis, as well as a target for up-regulation by three UPR pathways. The ER protein chaperone, the glucose-regulated protein 78 kDa (GRP78), plays a major role in the adaptive response to ER stress. The elevated expression of GRP78 plays a major role in the pro-survival and cytoprotective response of cancer cells to the stress.16 In addition to the change of intracellular Ca2+ level, the levels of CHOP and the GRP78 are usually altered in the presence of stress.16 Previous reports indicated that CHOP plays an important role in the ER stress-induced apoptosis.19 Both PERK and ATF6 can induce the expression of the transcription factor CHOP, which in turn leads to a reduction in the expression of anti-apoptotic Bcl-2 and the up-regulation of several proapoptotic genes.26 On the other hand, GRP78 is recruited to misfolded proteins to help protein folding under ER stress, which is important in the survival program.19 In our study, curcumin increased the mRNA and protein levels of CHOP, while no obvious change of GRP78 expression at the mRNA level was observed (Fig. 3). These data indicate that GRP78 involved in ER stress appears to be highly cell type and context dependent. ATF6, a key regulator of the UPR, is known to be important for ER stress-mediated apoptosis and cell growth.40 Upon ER stress, the accumulation of misfolded proteins in the ER results in the cleavage of ATF6 by serine protease site-1 protease (S1P) and metalloprotease site-2 (S2P), which are two proteases, locating at the Golgi compartment, cleaving ATF6 in the luminal domain and the N-terminal portion respectively. The transactivation domain of ATF6 will move to the nucleus and activate the transcription of ER chaperones.21,40 Recently studies indicated that ATF6 can enhance the expression of IRE1a-spliced XBP1s gene via regulating endogenous XBP1s gene.40 The splicing of XBP1 mRNA is required for IRE1α autophosphorylation. Then the splicing XBP-1 (XBP-1s) translocates into the nucleus, where it binds to its target chaperone genes to induce their transcription, including stress-associated endoplasmic reticulum protein 1 (SERP1), DnaJ/Hsp40-like genes, p58IPK, ERdj4, and HEDJ, as well as EDEM, protein disulfide isomerase-P5 and ribosome-associated membrane protein 4 (RAMP4).41 As the downstream of the XBP1, stress-associated endoplasmic reticulum protein 1 (SERP1) was induced under ER stress. Its overexpression stabilizes newly synthesized membrane proteins under ER stress by associating with the Sec61 complex.31 In our study, we found that curcumin induced the cleavage of ATF6 and enhanced the splicing of XBP1 in the BCPAP cells (Fig. 4). Moreover, the activated XBP1 up-regulated the mRNA level of its downstream target SERP1. These results showed that curcumin may activate ER stress related genes to recover ER homeostasis.
We found a novel way, the endoplasmic reticulum stress pathway, to induce the death of BCPAP cells by curcumin, which provided a new idea to study the mechanism of inhibiting the growth of the BCPAP cells by curcumin. But our study is confined to one type of thyroid carcinoma cell line, lacking some universal applicabilities. The effects of curcumin on the growth of other PTC cell lines or other cell lines derived from different types of thyroid cancers, including follicular thyroid cancer cell line FTC133 and anaplastic thyroid cancer cell line 8505C were in progress.
5. Conclusions
Collectively, our results indicated that curcumin inhibit cell growth and induce the ER stress in the papillary thyroid carcinoma cells via activating the ATF6/XBP-1 pathway. These findings suggest that curcumin may be a potential anticancer drug for thyroid carcinoma treatment.Acknowledgements
This study was supported by the grants from the Ministry of Health Foundation of China (W201304) and the National Natural Science Foundation of China (No. 81402214, 31471696 and 91313303).References
- Y. E. Nikiforov and M. N. Nikiforova, Nat. Rev. Endocrinol., 2011, 7, 569–580 CrossRef CAS PubMed.
- M. Niedziela, Best Pract. Res., Clin. Endocrinol. Metab., 2014, 28, 245–277 CrossRef CAS PubMed.
- R. M. Carneiro, B. A. Carneiro, M. Agulnik, P. A. Kopp and F. J. Giles, Cancer Treat. Rev., 2015, 41, 690–698 CrossRef CAS PubMed.
- J. J. Bright, J. Immunol., 2002, 168, 6506–6513 CrossRef.
- A. H. Rahmani, M. A. Al Zohairy, S. M. Aly and M. A. Khan, Biomed Res. Int., 2014, 2014, 761608 Search PubMed.
- S. Avasarala, F. Zhang, G. Liu, R. Wang, S. D. London and L. London, PLoS One, 2013, 8, e57285 CAS.
- D. Subramaniam, S. Ponnurangam, P. Ramamoorthy, D. Standing, R. J. Battafarano, S. Anant and P. Sharma, PLoS One, 2012, 7, e30590 CAS.
- P. H. Killian, E. Kronski, K. M. Michalik, O. Barbieri, S. Astigiano, C. P. Sommerhoff, U. Pfeffer, A. G. Nerlich and B. E. Bachmeier, Carcinogenesis, 2012, 33, 2507–2519 CrossRef CAS PubMed.
- C. Y. Zhang, L. Zhang, H. X. Yu, J. D. Bao, Z. Sun and R. R. Lu, Food Chem., 2013, 139, 1021–1028 CrossRef CAS PubMed.
- C. Y. Zhang, L. Zhang, H. X. Yu, J. D. Bao and R. R. Lu, Biotechnol. Lett., 2013, 35, 995–1000 CrossRef CAS PubMed.
- C. Tan, L. Zhang, X. Cheng, X. F. Lin, R. R. Lu, J. D. Bao and H. X. Yu, Exp. Biol. Med., 2015, 240, 925–935 CrossRef CAS PubMed.
- R. Sano and J. C. Reed, Biochim. Biophys. Acta, Mol. Cell Res., 2013, 1833, 3460–3470 CrossRef CAS PubMed.
- H. Wu, L. Wei, F. Fan, S. Ji, S. Zhang, J. Geng, L. Hong, X. Fan, Q. Chen, J. Tian, M. Jiang, X. Sun, C. Jin, Z. Y. Yin, Q. Liu, J. Zhang, F. Qin, K. H. Lin, J. S. Yu, X. Deng, H. R. Wang, B. Zhao, R. L. Johnson, L. Chen and D. Zhou, Nat. Commun., 2015, 6, 6239 CrossRef CAS PubMed.
- S. Lee, S. Min Kim, J. Dotimas, L. Li, E. P. Feener, S. Baldus, R. B. Myers, W. A. Chutkow, P. Patwari, J. Yoshioka and R. T. Lee, EMBO Mol. Med., 2014, 6, 732–743 CrossRef CAS PubMed.
- H. J. Kim, J. S. Jeong, S. R. Kim, S. Y. Park, H. J. Chae and Y. C. Lee, Sci. Rep., 2013, 3, 1142 Search PubMed.
- W. A. Wang, J. Groenendyk and M. Michalak, Biochim. Biophys. Acta, Mol. Cell Res., 2014, 1843, 2143–2149 CrossRef CAS PubMed.
- Q. Luan, L. Jin, C. C. Jiang, K. H. Tay, F. Lai, X. Y. Liu, Y. L. Liu, S. T. Guo, C. Y. Li, X. G. Yan, H. Y. Tseng and X. D. Zhang, Autophagy, 2015, 11, 975–994 CrossRef CAS PubMed.
- W. Li, Z. Ouyang, Q. Zhang, L. Wang, Y. Shen, X. Wu, Y. Gu, Y. Shu, B. Yu, X. Wu, Y. Sun and Q. Xu, Cell Death Dis., 2014, 5, e1581 CrossRef CAS PubMed.
- H. Q. Wang, Z. X. Du, H. Y. Zhang and D. X. Gao, Endocrinology, 2007, 148, 3258–3270 CrossRef CAS PubMed.
- L. Ulianich, C. Garbi, A. S. Treglia, D. Punzi, C. Miele, G. A. Raciti, F. Beguinot, E. Consiglio and B. Di Jeso, J. Cell Sci., 2008, 121, 477–486 CrossRef CAS PubMed.
- M. Zheng, Q. Zhang, Y. Joe, B. H. Lee, G. Ryu do, K. B. Kwon, S. W. Ryter and H. T. Chung, Int. Immunopharmacol., 2013, 15, 517–523 CrossRef CAS PubMed.
- A. Cao, Q. Li, P. Yin, Y. Dong, H. Shi, L. Wang, G. Ji, J. Xie and D. Wu, Apoptosis, 2013, 18, 1391–1402 CrossRef CAS PubMed.
- S. H. Wu, L. W. Hang, J. S. Yang, H. Y. Chen, H. Y. Lin, J. H. Chiang, C. C. Lu, J. L. Yang, T. Y. Lai, Y. C. Ko and J. G. Chung, Anticancer Res., 2010, 30, 2125–2134 CAS.
- A. I. Placido, C. R. Oliveira, P. I. Moreira and C. M. Pereira, Mol. Neurobiol., 2015, 51, 571–590 CrossRef CAS PubMed.
- F. Song, L. Zhang, H.-X. Yu, R.-R. Lu, J.-D. Bao, C. Tan and Z. Sun, Food Chem., 2012, 132, 43–50 CrossRef CAS PubMed.
- Y. L. Huang, C. L. Chang, C. H. Tang, Y. C. Lin, T. K. Ju, W. P. Huang and H. Lee, Cell. Signalling, 2014, 26, 611–618 CrossRef CAS PubMed.
- X. Zheng, X. Zheng, X. Wang, Z. Ma, V. Gupta Sunkari, I. Botusan, T. Takeda, A. Björklund, M. Inoue, S. B. Catrina, K. Brismar, L. Poellinger and T. S. Pereira, Cell Death Dis., 2012, 3, e322 CrossRef CAS PubMed.
- M. Schröder and R. J. Kaufman, Annu. Rev. Biochem., 2005, 74, 739–789 CrossRef PubMed.
- S. Kim, Y. Joe, H. J. Kim, Y. S. Kim, S. O. Jeong, H. O. Pae, S. W. Ryter, Y. J. Surh and H. T. Chung, J. Immunol., 2015, 194, 4498–4506 CrossRef CAS PubMed.
- O. H. Atsushi Yamaguchi, D. M. Stern, E. Hartmann, S. Ogawa and M. Tohyama, J. Biol. Chem., 1999, 147, 1195–1204 Search PubMed.
- O. Hori, M. Miyazaki, T. Tamatani, K. Ozawa, K. Takano, M. Okabe, M. Ikawa, E. Hartmann, P. Mai, D. M. Stern, Y. Kitao and S. Ogawa, Mol. Cell. Biol., 2006, 26, 4257–4267 CrossRef CAS PubMed.
- S. J. Mandel, L. K. Shankar, F. Benard, A. Yamamoto and A. Alavi, Clin. Nucl. Med., 2001, 26, 1–9 CrossRef.
- S. Monzen, Y. Mariya, A. Wojcik, C. Kawamura, A. Nakamura, M. Chiba, M. Hosoda and Y. Takai, Mol. Clin. Oncol., 2015, 3, 692–698 Search PubMed.
- R. T. Anderson, J. E. Linnehan, V. Tongbram, K. Keating and L. J. Wirth, Thyroid, 2013, 23, 392–407 CrossRef CAS PubMed.
- Z. Meng, S. Lou, J. Tan, K. Xu, Q. Jia and W. Zheng, PLoS One, 2012, 7, e33597 CAS.
- K. W. Kim, L. Moretti, L. R. Mitchell, D. K. Jung and B. Lu, Oncogene, 2010, 29, 3241–3251 CrossRef CAS PubMed.
- E. J. Sherman, Y. B. Su, A. Lyall, H. Schoder, M. G. Fury, R. A. Ghossein, S. Haque, D. Lisa, A. R. Shaha, R. M. Tuttle and D. G. Pfister, Thyroid, 2013, 23, 593–599 CrossRef CAS PubMed.
- M. J. Tuorkey, Interventional Medicine and Applied Science, 2014, 6, 139–146 CrossRef PubMed.
- M. Hoyer-Hansen and M. Jaattela, Cell Death Differ., 2007, 14, 1576–1582 CrossRef CAS PubMed.
- X. Han, P. Zhang, R. Jiang, F. Xia, M. Li and F. J. Guo, Histochem. Cell Biol., 2014, 142, 497–509 CrossRef CAS PubMed.
- A. H. Lee, N. N. Iwakoshi and L. H. Glimcher, Mol. Cell. Biol., 2003, 23, 7448–7459 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |